By Diana Le, Pashyn Morimoto and Bradley O. Ashburn
Abstract
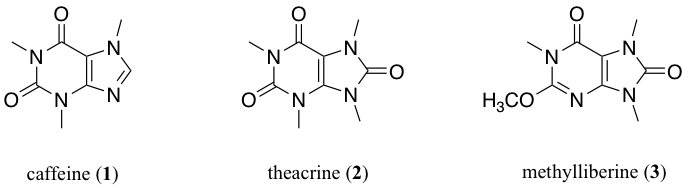
Nutritional supplements comprised a $123 billion industry globally in 2019 with energy and cognitive enhancement products constituting a quarter of that amount. However, these products are not regulated by the Food and Drug Administration (FDA) and manufacturers typically make bold, unsubstantiated claims of product efficacy. Unbiased, neutral-party evaluation is vital for public health safety. The goal of this study was to model the absorption of three energy-enhancing, nootropics (caffeine (1), theacrine (2), and methylliberine (3)) through the use of an octanol/water mixed solvent system and UV-Vis spectrophotometry to determine their lipophilicity. The measure of a molecule’s lipophilicity is a valuable pharmacological and toxicological property which is used to determine how well a molecule is absorbed through cellular membranes from extracellular fluid into the cell. Standard curves of known concentrations were created using serial dilution to enable analysis of the unknown concentration after partitioning. The resulting values were replicated by duplicate trials for validation of reproducibility.
Introduction
Caffeine and other dietary supplements advertised to increase energy and alertness are widely used by the general population.1,2,3 Caffeine is consumed by 89% of adults in the US,4 and 85% of the US population consumes at least one caffeinated drink per day.5 As an ergogenic aid, caffeine is used by athletes, military personnel, and others who seek improved physical and mental performance.1,3,6 The mechanism of action involves binding to adenosine receptors in the brain resulting in the prevention of adenosine reuptake that would normally trigger tiredness.7 The result is increased alertness and enhanced ability to maintain attention.
More recently, supplements containing theacrine and methylliberine, purine alkaloids found naturally in the leaves of the Camellia Kucha plant, have gained popularity among those seeking to increase energy and to improve focus, concentration, and mood. Though not as extensively studied as caffeine, early evidence does support the efficacy of theacrine- and methylliberine-containing supplements in improving subjective measures related to energy, mood, and fatigue reduction.8,9 Safety data with theacrine supplementation indicates when taken as directed, the compound is safe and not shown to adversely affect physiological indicators such as heart rate, blood pressure, rate pressure product, or other clinical safety measures.9,10 Given the widespread use of caffeine and other energy supplements, the need for more studies into the efficacy and physicochemical properties of these compounds is needed to ensure public safety as the supplement industry is not regulated by the FDA. This study experimentally determines the valuable chemical property known as the partition coefficient for all three supplements: caffeine, theacrine, and methylliberine. The partition coefficient (P) is a measure of the solubility of a compound in a water/1-octanol mixed solvent system. It is mathematically defined as the ratio of the concentration of the non-ionized compound at equilibrium between organic and aqueous phases.This system is widely used by medicinal chemists as a model for cellular absorption.Taking the logarithm of the partition coefficient (logP) provides a simple value to indicate a compound’s lipophilicity. Thus, logP has various applications such in drug discovery, agrochemicals, flavors, and fragrances.
Methods and Materials
Chemical reagents were purchased from Sigma-Aldrich or directly from the manufacturer. A Perkin Elmer LambdaXLS+ UV-Vis spectrophotometer was used for assay.
Standard Curve: Stock solutions of caffeine, theacrine and methylliberine were prepared with deionized water at a concentration of 100 μg/ml for each compound. Serial dilution of each stock solution was used to obtain concentrations of 5, 10, 15, 20, 25, and 30 μg/ml in order to create standard curves of concentration vs UV absorbance. The maximum absorbance (λmax) of caffeine (274 nm), theacrine (274 nm), and methylliberine (284 nm) were established by measuring the stock solution over a range of 200-800 nm wavelengths. Deionized water was used as a blank to zero the spectrophotometer before measurement of each sample. The data is reported in Tables 1-3 and standard curves in Figures 1-3.
Partitioning Experiment: Equal parts 1-octanol (10 mL) and supplement (10 mL, 20 ul/mL) were added to an Erlenmeyer flask. A 1:1 ratio of 1-octanol and water was prepared as the blank. Each solution was vigorously stirred at room temperature for 24 hours. The mixtures were then allowed to equilibrate into two phases for a subsequent 24-hour period. A sample of the aqueous phase of each mixture was measured via UV spectrophotometry at the respective λmax. The experiment was repeated in triplicate and the median value used.
Results Figure 2 shows the standard curve for caffeine at its λmax of 274 nm. There is a linear relationship between absorbance and concentration with a strong correlation of 0.9994. Using statistical analysis, a linear regression formula was found to be f(x) = 0.0477x + 0.1286. This equation provides the means to analyze the unknown concentration of caffeine upon partitioning in the mixed 1-octanol/water solvent system.
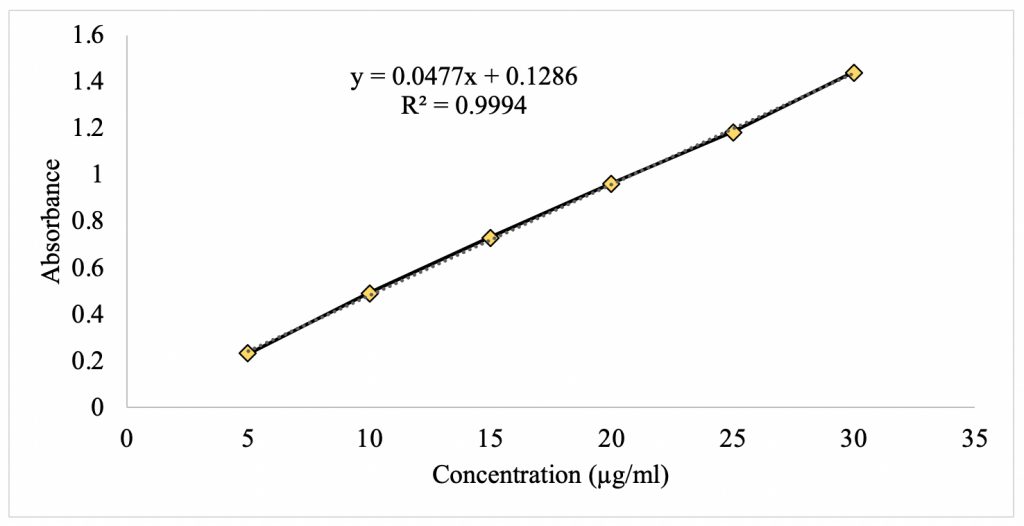
Figure 3 shows the standard curve for theacrine at its λmax of 274 nm. There is a linear relationship between absorbance and concentration with a strong correlation of 0.9996. Using statistical analysis, a linear regression formula was found to be f(x) = 0.02155x – 0.02053. This equation provides the means to analyze the unknown concentration of caffeine upon partitioning in the mixed 1-octanol/water solvent system.
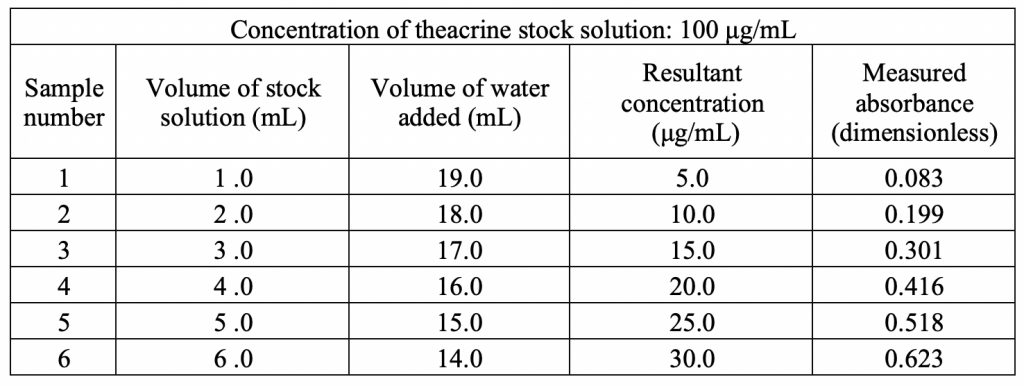
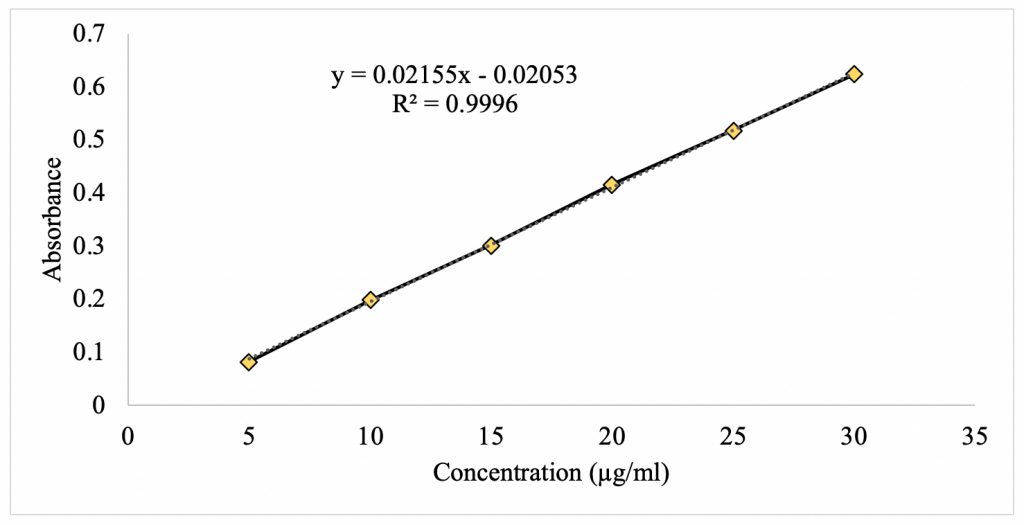
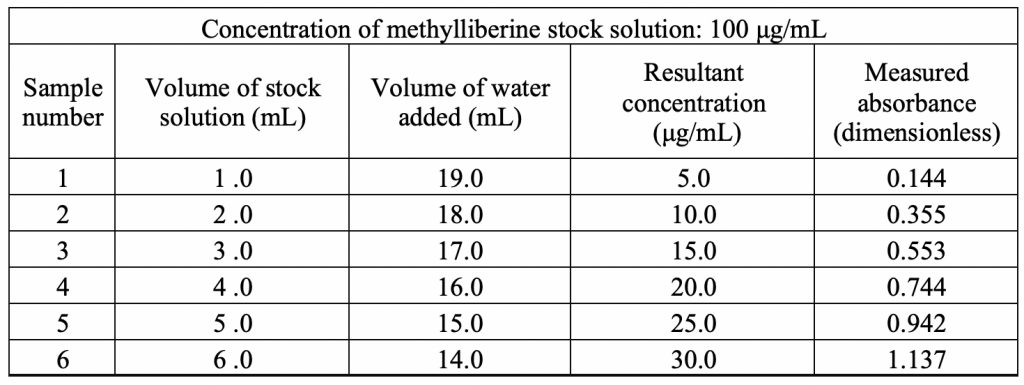
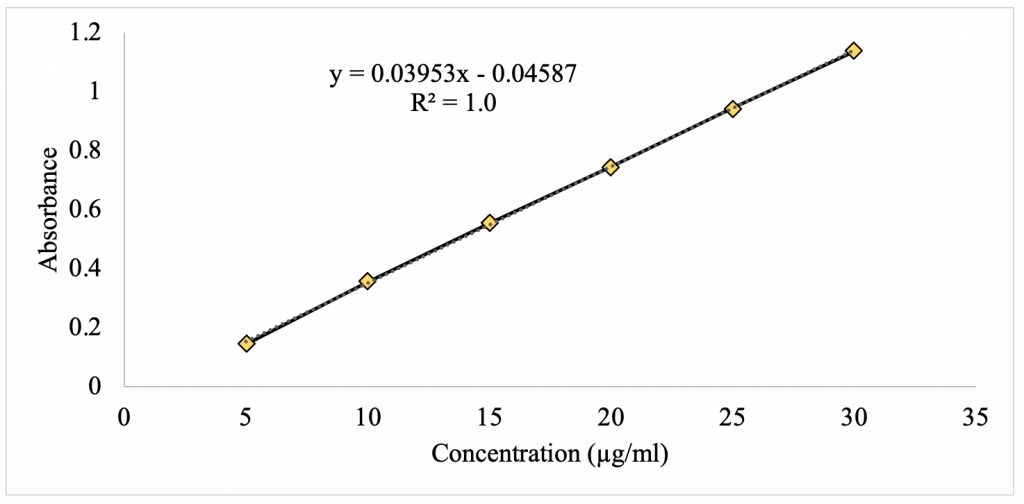
Figure 4 shows the standard curve for methylliberine at its λmax of 284 nm. There is a linear relationship between absorbance and concentration with a strong correlation of 1.0. Using statistical analysis, a linear regression formula was found to be f(x) = 0.03953x – 0.04587. This equation provides the means to analyze the unknown concentration of caffeine upon partitioning in the mixed 1-octanol/water solvent system.
Following the partitioning procedure outlined in the Methods section, Table 4 summarizes the data generated for each compound. The unknown concentration of the aqueous layer was measured for UV absorbance and using the equation from the linear regression of the standard curve, the concentration of the compound in the aqueous layer was determined. Since the initial concentration of each compound was known, the concentration in the 1-octanol layer was calculated. Taking the logarithm of the ratio of the concentration of compound in water divided by the concentration in 1-octanol yields the partition coefficient (logP).
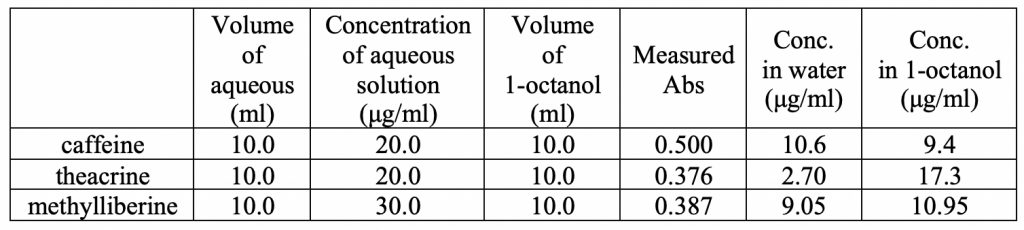
The logP for caffeine was -0.07, the logP for theacrine was -1.06, and the logP for methylliberine was -0.083. The literature value of logP for caffeine is -0.06 which validates our experimental technique. These results show that theacrine is more hydrophilic, which can be rationalized by the addition of the carbonyl oxygen compared to caffeine. Comparing theacrine to methylliberine, it was found that methylliberine has reduced hydrophilicity. This is explained by conversion of the more polar carbonyl group into a less polar methyl enol ether. These differences begin to provide a basis for understanding how they are absorbed and distributed within our bodies.
Conclusion
The discovery of alternative compounds for enhanced energy, mood, and alertness is of great importance to society. Considering the negative side effects of prolonged caffeine addiction, the food and fitness supplementation industry is actively promoting new products containing theacrine and methylliberine. This study elucidates valuable physicochemical properties of these compounds for future in vivo study by the scientific community.
Conflict of Interest
The authors report no conflicts of interest. No funding was provided by the manufacturer of the nutritional supplements theacrine or methylliberine.
Acknowledgements
This project was supported by grants from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS), IDeA Networks of Biomedical Research Excellence (INBRE), Award number: P20GM103466. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
[1] Lieberman, H.R., Marriott, B.P., Williams, C., Judelson, D.A., Glickman, E.L., Geiselman P.J., Dotson, L. and Mahoney, C.R. (2015) Clinical Nutrition, 34, 976-985.
[2] Dickinson, A., Blatman, J., El-Dash, N. and Franco, J.C. (2014) Journal of the American College of Nutrition, 33, 176-182.
[3] McLellan, T.M., Riviere, L.A., Williams, K.W., McGurk, D. and Lieberman, H.R. (2018) Nutritional Neuroscience.
[4] Fulgoni III, V.L., Keast, D.P. and Lieberman, H.R. (2015) The American Journal of Clinical Nutrition, 101, 1081-1087.
[5] Mitchell, D.C., Knight, C.A., Hockenberry, J., Teplansky, R. and Hartman, T.J. (2014) Chemical Toxicology, 63, 136-142.
[6] Del Coso, J., Muñoz, G. and Muñoz-Guerra, J. (2010) Applied Physiology, Nutrition, and Metabolism, 36, 555-561.
[7] Heckman, M.A., Weil, J. and Mejia, E.G.D. (2010) Journal of Food Science, 75, 77-87.
[8] Habowski, S.M., Sandrock, J.E., Kedia, A.W. and Ziegenfuss, T. (2014) Journal of the International Society of Sports Nutrition, 11, 49-50.
[9] Kuhman, D.J., Joyner, K.J. and Bloomer, R.J. (2015) Nutrients, 7, 9618-9632.
[10] Taylor, L., Mumford, P., Roberts, M., Hayward, S., Mullins, J., Urbina, S. and Wilborn, C. (2016) Journal of the International Society of Sports Nutrition, 13, 1-14.
About the Authors
Diana Le is a third-year student at University of Hawai‘i West O‘ahu majoring in health professions with a focus in pre-physician assistant. Born and raised in the culturally vibrant state of Hawai‘i, she is the youngest of four siblings, and is a product of first-generation college students. Her goal is to increase diversity in the healthcare field through practicing compassion, cultural competency, and continually improving the quality of life for individuals on their journey towards optimum health.
Pashyn was born and raised on the island of O‘ahu and is a graduate of Kamehameha Schools Kapālama, which takes pride in educating students about their Hawaiian ancestry. She is a first- generation college student majoring in health professions with a concentration in pre-medical studies. Her future plans include medical school and traveling around the globe to gain various perspectives and knowledge from many cultures. Her current interest is focused on chronic diseases and preventative healthcare. Pashyn’s ultimate goal is to live a life full of aloha and to make a lasting, positive, impact on each individual she encounters.
Bradley O. Ashburn is assistant professor of chemistry at the University of Hawaii West O’ahu.
• • •
Enjoyed this story? Enter your email to receive notifications of new posts by email
