By Nicholas Bailey, Pashyn Morimoto, Helmut Kae, Bradley O. Ashburn
Abstract
Pulmonary aspergillosis is an escalating issue for immunocompromised individuals. The mortality rates range from 40-90% with approximately three million cases of chronic pulmonary aspergillosis worldwide annually. Current treatment options are becoming less effective due to a rise in anti-fungal resistant strains; thus, it is imperative that novel medicines be developed to alleviate those suffering from this disease. Chalcones and imidazoles are two pharmacophores that have a wide range of biological activities. Our objective was to combine these two pharmacophores in a novel way and test the effectiveness against three fungal species. The chalcones were synthesized via the Claisen-Schmidt aldol condensation of 4-(1H-Imidazol-1-yl)benzaldehyde with various substituted acetophenones using aqueous sodium hydroxide in methanol. Following this, the compounds were tested for anti-fungal properties via a well-diffusion assay against Aspergillus fumigatus, Aspergillus niger, and Candida albicans. The data obtained suggested that two of the three chalcones synthesized were moderately effective against all species of fungi tested. The fluorinated chalcone appeared to be the most promising with potential to act as a therapeutic for treatment of pulmonary aspergillosis.
Introduction
Fungal diseases are a neglected area in current biomedical research despite mortality associated with fungal disease affecting more than 1.6 million people annually (similar to tuberculosis) and three-fold more than malaria (1). A specific genus of fungus, Aspergillus, is found all over the world with over 200 species having been described. Aspergillus species can cause a range of diseases known as aspergillosis. There are multiple etiological agents of aspergillosis (2); however, the main fungus of interest in the current study is Aspergillus fumigatus becauseit is the primary species that causes illnesses in humans (3). Inhalation of the Aspergillus spores, also known as conidia, is the most common way individuals are infected. The primary route of infection of Aspergillus is through the respiratory tract since the conidia can bypass mucociliary clearance and lodge in the lower respiratory tract (3,4); however, other tissues like the skin, sinuses, central nervous system, eyes, and nails can be infected as well (5). Symptoms associated with infection of Aspergillus can develop into more serious conditions and can even be fatal.
Immunocompromised individuals who have had treatments such as stem cell and solid organ transplants are most at risk for infection from Aspergillus fungi (6), as are populations include those with previous lung conditions or chronic diseases. Mortality rates from aspergillosis can range anywhere from 40-90% depending on the health of the individual (3). The incidence of aspergillosis has risen four-fold in the last 13 years (5), and cases may continue rising as long as there are limited drug therapies, antifungal resistant strains, and a growth of individuals with compromised immune systems. Current first-line agents for the treatment of aspergillosis are azoles which include voriconazole and isavuconazole; however, resistance to these agents is an emerging threat (7,8). In addition, recent studies have shown that severe respiratory infections such as influenza and coronavirus disease 2019 (COVID-19) can lead to secondary infection of aspergillosis (9) and these secondary infections of COVID-19/influenza-associated aspergillosis have been connected to higher mortality rates (10). Development of new and safe treatments may help to provide potential medications to individuals with aspergillosis.
Chalcones are molecules that possess many pharmacological properties such as antimalarial, antiprotozoal, anti-inflammatory, anticancer, antibacterial, and antidiabetic properties (11-13). Hussain demonstrated that adding various substituents, such as imidazole rings, to the aromatic rings of chalcones has potential for obtaining pharmacologically active compounds (14). The aim of this project is to synthesize and evaluate the effectiveness of imidazole-bearing chalcones with various substituents against numerous species of fungi. We hypothesize that results from this study will show that chalcones are effective at inhibiting the growth of numerous fungal species, and therefore encourage further research on the use of synthesized chalcones as potential treatments for those suffering from fungal infections.
Methods
Synthesized chalcones were tested against Aspergillus niger, Candida albicans, and Aspergillus fumigatus. A. niger and C. albicans were used to optimize the testing methodology for the effectiveness of previously synthesized chalcones before transitioning to A. fumigatus.
Assay medium and microdilution plates
Fungi were maintained and passaged on PDA agar. YPD broth was used for the microdilution assays.
Preparation of the inoculum
The A. niger fungal strain was obtained from the American Type Culture Collection (ATCC) and C. albicans and A. niger strains were obtained from Carolina Biologicals. Inoculum suspensions for all three fungi were prepared from two five-day-old cultures grown on potato dextrose agar (PDA) slants at 30ºC. The inoculum was prepared by collecting spores of the cultures with a sterile swab and suspending the spores in a solution of 10 mL sterile water with 0.01% Triton X-100. The spore suspension for A. fumigatus did not contain Triton, only sterile water. The suspension was homogenized by vortexing for approximately five seconds (15). The opacity of the suspension was adjusted using a spectrophotometer (Thermo Scientific Genesys 10vis) that measured the absorbance of the suspended spores solution at 600 nm. The range of absorbance desired was between 0.09-0.13 (16).
Preparation of the treatments
Three chalcones were utilized in this study: (E)-1-(4-fluorophenyl)-3-[4-(1H-imidazol-1-yl)phenyl]prop-2-en-1-one (chalcone 3a), (E)-1-(4-bromophenyl)-3-[4-(imidazol-1-yl)phenyl]prop-2-en-1-one (chalcone 3b), and (E)-3-[4-(imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one (chalcone 3c). A positive control, Amphotericin B, and a negative control, DMSO, were also used. Chalcones 3a, 3b, and 3c were put into solution using the solvent DMSO. A serial dilution method was used to dilute the chalcones and Amphotericin B to obtain initial concentrations of 2000 μg/ml, 1000 μg/ml, 500 μg/ml, 200 μg/ml, and 100 μg/ml.
Inoculation of microdilution plates
96-well plates were used to conduct this experiment. To each well, 100 µl of 2-times concentrated YPD broth, 90 µl of spore suspension, and 10 µl of the respective treatment was added. Final concentrations of the treatments were 100 μg/ml, 50 μg/ml, 25 μg/ml, 10 μg/ml, 5 μg/ml. Each of the dilutions of chalcones 3a, 3b, and 3c, and Amphotericin B (positive control) were done in triplicate. 10 µL DMSO was used as a negative control. An initial absorbance reading was done for the microdilution plates by a spectrophotometer (BIO-RAD Model 680 Microplate Reader) at 650 nm. The microdilution plates for A. niger and C. albicans were incubated at 20ºC (room temperature) for approximately 72 hours before analysis; A. niger was incubated for approximately 48 hours. Analysis of the chalcone’s effectiveness to kill fungi was determined using this absorbance information gained by a spectrophotometer reading.
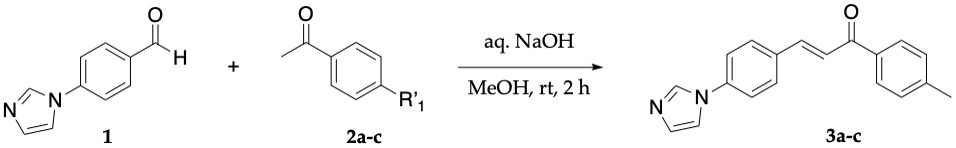
Synthesis and Characterization of Chalcones Chalcones 3a-3c were synthesized via a Claisen-Schmidt condensation (Figure 1). The reaction was performed by adding 4-(1H-Imidazol-1-yl)benzaldehyde 1, a select acetophenone 2a-2c, and methanol to a round bottom flask at room temperature. Aqueous NaOH was added and allowed to stir for two hours. The crude products were recrystallized in hot methanol and obtained in good yield. Confirmation of known target chalcones (3a and 3b) was confirmed using IR (ThermoFisher iS5 FT-IR) and melting point (Stuart SMP3 melting point apparatus). The structure of the novel chalcone 3c was additionally verified using ¹H NMR, ¹³C NMR (500 MHz Bruker AV-500 NMR spectrometer), and electrospray ionization mass spectrometry (Perkin Elmer PE-SCIEX API-150).
Results
Synthesis and Characterization of Chalcones
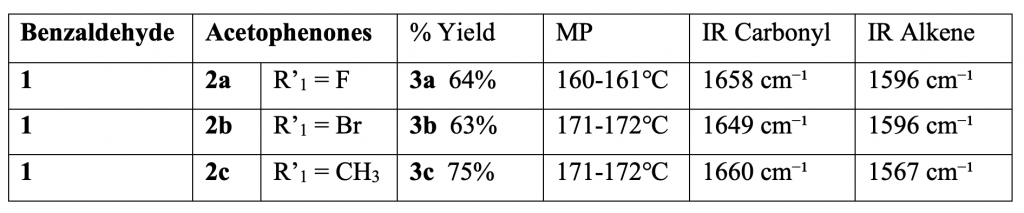
Chalcones 3a-3c were obtained in the following yields: 64%, 63%, and 75% respectively. All chalcones exhibited a narrow melting point range and characteristic IR peaks of the conjugated ketone moiety and the alkene indicated that we had successfully synthesized our target chalcones (Table 1).
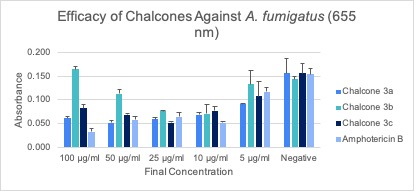
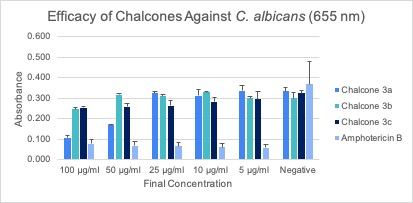
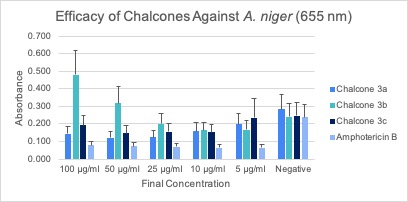
Microdilution Assay After the respective incubation time for each assay, microdilution assays were subjected to an absorbance reading using a spectrophotometer and then analyzed, and the average of the triplicates was calculated (Figures 2A-C). Error bars represent the standard deviation of the mean.
Discussion
After analyzing the data and trends displayed from the figures for each of the assays, chalcone 3a appears to be the most effective against all three fungal species (Figures 2A-C). For chalcone 3a, the trend of higher concentrations being more effective is most apparent in the C. albicans and A. fumigatus assays. The data obtained for chalcone 3b was deemed to be inconclusive due to the inconsistency of values gained from the spectrophotometer readings. Chalcone 3c is possibly effective against fungal species, however more experimentation on this chalcone would have to be completed to confirm its true efficacy. None of the chalcones proved to be more effective than the positive control, Amphotericin B. However, chalcone 3a showed similar activity to Amphotericin B when tested against A. fumigatus.
Absorbance readings could have been affected by each species’ pattern of growth. C. albicans and A. fumigatus propagated evenly within the wells of the assay, allowing for uniform growth within the wells. However, A. niger appeared to grow along the walls of the well, allowing light to pass through the center when absorbance readings were being done, and therefore may have impacted readings. Consequently, the absorbance readings from A. niger may have not illustrated the full scope of growth for this fungus.
Additionally, absorbance readings could have been affected by the addition of the surfactant, Triton X-100. Triton X-100 was combined with sterile water during the preparation of the inoculum for C. albicans and A. niger. The initial purpose was to keep the largely hydrophobic spores from clumping together, facilitating the enumeration process. However, Triton X-100 caused the formation of bubbles when pipetting the inoculum into the wells. These bubbles could have altered the values of absorbance.
When pipetting the chalcones into the wells at a final concentration of 100 µg/ml, an observed cloudiness formed. This could be attributed to the formation of a precipitate due to reduced solubility. Consistent with this observation was the fact that cloudiness was only detected in this higher concentration of chalcones. This cloudiness could have affected the absorbance readings of those wells because it would alter the opacity of the solution within the well. It is hypothesized that the concentration of the treatment was too high, which is why it precipitated out in the low volume environment of the assay wells.
Conclusion
The data obtained from this study suggests that imidazole-bearing chalcones are effective as antifungal treatments. Chalcone 3a in particular appears to be the most promising chalcone with potential to act as a therapeutic for treatment of aspergillosis. The data also suggests that imidazole bearing chalcones with fluorine atoms, like chalcone 3a, may have a greater effect on killing microbes than other variations of chalcones. Thus, further syntheses of imidazolyl-bearing chalcones with fluorine atoms at different positions should be performed to test if the positioning of the electron-withdrawing group plays a role in the effectiveness of killing fungal species.
Through multiple trials, researchers learned that the optimal method for this study is the microdilution assay. Therefore, future studies should place an emphasis on using this method to identify potential drugs that inhibit A. fumigatus due to its adverse health effects on humans. Replication and further optimization of the assay with the main fungus of interest will solidify results obtained during this study; researchers hope to gain statistically significant evidence for chalcone effectiveness against fungi. This paper describes a small portion of a long-term study that will continue to grow for years to come.
References
- Bongomin, F., Gago, S., Oladele, R.O., Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi, 2017, 3, 57.
- Rudramurthy, S.M., Paul, R.A., Chakrabarti, A., Mouton, J.W., Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi, 2019, 5, 55.
- Dagenais, T., Keller, N. Pathogenesis of aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev., 2009, 22, 447-465.
- Tischler, B.Y., Hohl, T.M. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. J. Mol. Biol., 2019, 431, 4229–4246.
- Vuong M.F., Waymack J.R. (2020) Aspergillosis, StatPearls Publishing.
- Centers for Disease Control and Prevention. (2019) Fungal Diseases, Types of Fungal Diseases, Aspergillosis – aspergillosis statistics.
- Jenks, J., Hoenigl, M. Treatment of Aspergillosis. J. Fungi, 2018, 4, 98.
- Romero, M., Messina, F., Marin, E., Arechavala, A., Depardo, R., Walker, L., Santiso, G. Antifungal Resistance in Clinical Isolates of Aspergillus spp.: When Local Epidemiology Breaks the Norm. J. Fungi, 2019, 5, 41.
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Severe Coronavirus Disease. Emerg. Infect. Dis., 2021, 27, 18-25.
- Waldeck, F.; Boroli, F.; Suh, N.; Garcia, P.D.W; Flury, D.; Notter, J.; Iten, A.; Kaiser, L.; Schrenzel, J.; Boggian, K.; Maggiorini, M.; Pugin, J.; Kleger, G.; Albrich W.C. Influenza Associated Aspergillosis in Critically-Ill Patients a Retrospective Bicentric Cohort Study. Eur. J. Clin. Microbiol., 2020, 39, 1915-1923.
- Hussain, T., Siddiqui, H.L., Zia-ur-Rehman, M., Masoom Yasinzai, M., Parvez, M. Anti-oxidant, anti-fungal and Anti-leishmanial activities of novel 3-[4-(1H-IMIDAZOL-1-YL) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem., 2009, 44, 4654-4660.
- Amato-Ocampo, J.; Carrillo, R.; Kae, H.; Ashburn, B.O. “Synthesis and Antimicrobial Evaluation of a Series of Chlorinated Chalcone Derivatives.” Int. Journal of Pharmacy and Pharmaceutical Research, 2018, 13, 112.
- Bailey, N.; Atanes, A.; Ashburn, B.O. (E)-3-(4-Chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one. Molbank, 2021, 2021, M1184.
- Hussain, T., Zia-ur-Rehman, M., Zaheer, M., Ashraf, C.M., Bolte, M. 1-[4-(1H-Imidazol-1-Yl)Phenyl]-3-Phenylprop-2-En-1-Ones – a potential pharmacophore bearing anti-leishmanial activity. J. Chem. Res., 2016, 40, 199-204.
- Lass-Flörl, C., Cuenca-Estrella, M., Denning, D.W., Rodriguez-Tudela, J.L. Antifungal susceptibility testing in Aspergillus spp. according to EUCAST methodology. Med. Mycol., 2006, 44, 319–325.
- Gupta, P., Khare, V., Kumar, D., Ahmad, A., Banerjee, G., Singh, M. Comparative evaluation of Disc diffusion and E-test with Broth Micro-dilution in Susceptibility testing of Amphotericin B, voriconazole and caspofungin against clinical Aspergillus isolates. J. Clin. Diagn, 2015, 9, 4-7.
About the Authors

Nicholas Bailey was born in 1995 on the island of O’ahu and is a proud graduate of the University of Hawai’i West O’ahu. He has remained at the university to complete additional coursework on his journey to becoming a psychiatrist. He believes in the statement, “a shared pain is half the pain and a shared joy is double the joy.” He knows that the cathartic nature of therapy cannot fix everything and that for some psychiatric medications may be the only solution. However, he would like his patients to be more informed about the dangers associated with long-term use. It is his belief that some of these psychiatric medications only treat symptoms and not the underlying cause. When he becomes a physician, he plans to prescribe medication only for short periods of time, and reinforce his prescriptions with therapy.

Pashyn Morimoto was born and raised on the island of O‘ahu, and will be graduating from the University of Hawai‘i West O‘ahu with a BAS in Health Professions Pre-Medical in December 2021. She is a first-generation college student with a career goal to become a Doctor of Osteopathic Medicine (D.O.). Her future plans include attending medical school and traveling around the globe to gain various perspectives and knowledge from many cultures. She strives to apply a patient-centered model to her future practice and to create a healthier community. Pashyn believes that educating people on how their personal lifestyle habits play such a significant role in their well-being and providing them with the resources to grow will gradually shift community health in a positive direction.
Helmut Kae is a professor of microbiology at Leeward Community College.
Bradley O. Ashburn is an associate professor of chemistry at the University of Hawaii West O’ahu.
• • •
Enjoyed this story? Enter your email to receive notifications.
