By Abby DeCoteau
United Tribes Technical College

Introduction
Owls are a common sight. Chances are you have seen them perched in trees gazing for prey or caught the quick and silent flash of an owl at night swooping down to capture its prey. Throughout the world, there are many different species of owl, ranging from the tiny palm-sized saw-whet owl (Aegolius acadicus) located in North America to the eagle-sized blakiston’s fish owl (Bubo blakistoni) located in Russia, China, and Japan. The most common owl found in North America is a larger species called the great horned owl (Bubo virginianus).
Great horned owls inhabit environments from northern Canada to southern South America (Tomazzoni, Pedó and Hartz 2004). They are good at adapting to their environment and can learn how to hunt in any habitat. In order to survive, owls have to either find a habitat containing natural food resources or change their feeding ecology (Zalewski 1994). They can inhabit many environments and adapt to preying on the most abundant species. Owls are year-round hunters and vary their prey selections during the winter and summer seasons (Kullberg 1995). It is becoming common to see predatory birds inhabiting urban areas, where they serve as important links in the urban food web (Zalewski 1994).
The diet of owls as a group is not well documented because they are mostly nocturnal and often occur in remote forested areas (Johnsgard 1988). Most research on owl diets is done by pellet analysis. Pellets are regurgitated parts of the prey that cannot be digested, such as bones, feathers, fur, and scales that the owl produces and discards. They can usually be found on the ground next to the owl roost (Yom-Tov and Wool 1997), a location where the owl chooses to perch and rest with maximum concealment during the daylight hours such as large trees or buildings (Johnsgard 1988). The pellets are collected then dissected.
There are two methods of pellet dissection. The first consists of thawing and drying the pellets, taking their measurements, then teasing them apart to reveal the prey items. The prey can then be identified, numbered, recorded, and stored in small paper envelopes. A second method involves the use of chemicals. Dry pellets are measured, then placed individually in jars and covered with a solution of potassium hydroxide. After the contents of each jar have soaked for 12 hours, they are poured into a fine mesh sieve and rinsed with fresh water to remove the dissolved hair (Dawe et al., 1978). Small mammals and birds are recognized by the cranial, mandibular, skull and bone remains, following keys provided by Chaline et al. (1974), Erome (1982) and, for birds, Aulagnier (1982).
Many different studies have utilized these techniques to examine owl diets. However, despite broad knowledge of owl diets, little is known of their prey in the vast northern Great Plains of midcontinent North America. Numerous studies of owl diets have been conducted in more wooded habitats of nearby Great Lakes states (Errington et al. 1940; Orians and Kuhlman 1956; Petersen 1979) and the boreal forest ecotone (McInvaille and Keith 1974; Rusch et al. 1972), but implications for predator-prey relationships in the Great Plains are only speculative (Murphy 1997). Although there have been multiple studies of owl diets, there has been no documented study on owl diets in the Bismarck, North Dakota area. Understanding owl diets in this region is critical because these birds of prey are a valuable source of pest control and help keep rodent populations down without the use of chemicals (Zabel, McKelvey and Ward 1995). With more information on the great horned owl and their diets we can better implement management strategies for both the prey and the owl. Bismarck also offers many different habitat types and owls may occupy most of them.
Hα: There will be a significant difference between a nesting owl pair diet at one site compared to a fledgling owl pair diet at another site.
Hο: There will not be a significant difference between a nesting owl pair diet at one site compared to a fledgling owl pair diet at another site.
Material and Methods
Study Areas
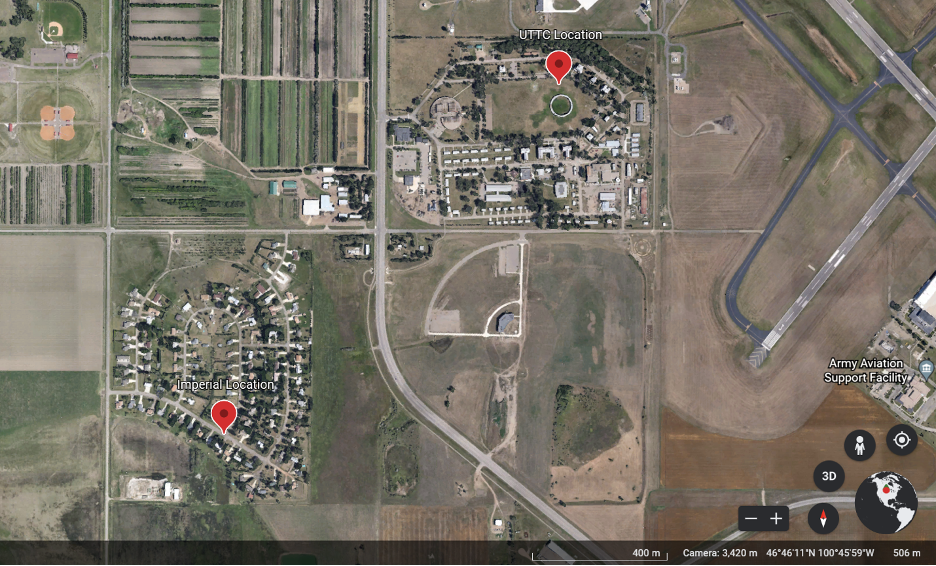
This research was carried out in two different locations in the Bismarck area. The first location was the campus of United Tribes Technical College (UTTC), which is located in the southern part of Bismarck. This area is urbanized with an abundance of large trees, open fields, and large old buildings. The second location was Imperial Drive, also located in the southern part of Bismarck. This area is an urbanized neighborhood adjacent to a wide-open field with scattered large trees. Both locations have an abundant human population mixed with quiet secluded areas where great horned owls can nest or roost.
Owl Pellet Collection
With permission to access these locations, I first went to the Imperial site to collect old pellets from below a nest no longer being used by a family of great horned owls. For my UTTC site I visited at dusk and before dawn. At dawn (5 am–8 am), using the reed R2160 thermal image camera, I would locate the owls and follow them (keeping a safe distance as to not make them uncomfortable with my presence) until they would find a roosting spot to rest. At dusk (7 pm – 9 pm) I returned to the roosting site. Once confirmed that the owl was gone, I searched the ground below for any pellets it regurgitated during the day. When pellets were found I documented specific locations using GPS, number of pellets found, dates, and times in my field journal. Using gloves and plastic bags I collected the pellets. One pellet was placed in its own individual bag and marked with the specific pellet number, location, date, and time. The pellets were then stored in a freezer until the day of examination. Freezing them also helped kill any larvae that might be present.
Owl Pellet Examination
Once in the lab, pellets were removed from the freezer and thawed for at least six hours. Once thawed, we would start examination of individual pellet content. Some pellets were so tightly compacted that they required soaking in warm water for one hour to separate fur from bones. For the pellets that were not as tightly compacted, we extracted contents without soaking the pellets. Examinations were done by separating prey remains (skulls, bones, feathers, etc.) from the rest of the pellet using forceps and tweezers. Species identification was done based on morphology and size of skulls and molars of rodents using mammal (Courant et al., 1997) and bird (Barbosa et al., 1990) bone key guides. Numerical and categorical data were collected to determine types and quantity of prey. The statistical test that I used to determine the differences in categorical data between groups was the chi-squared test. I then determined the differences between prey amongst the study sites and types (mammals and avian).
Results
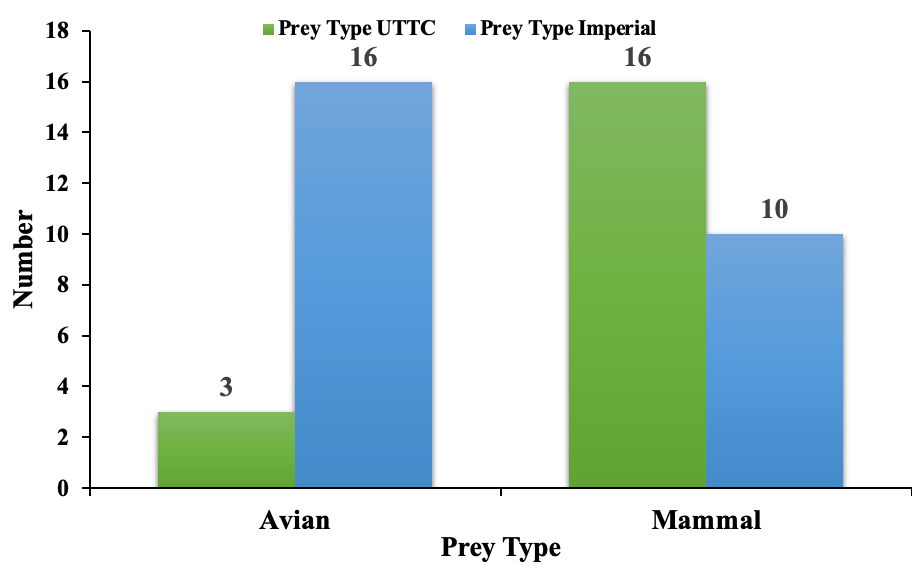
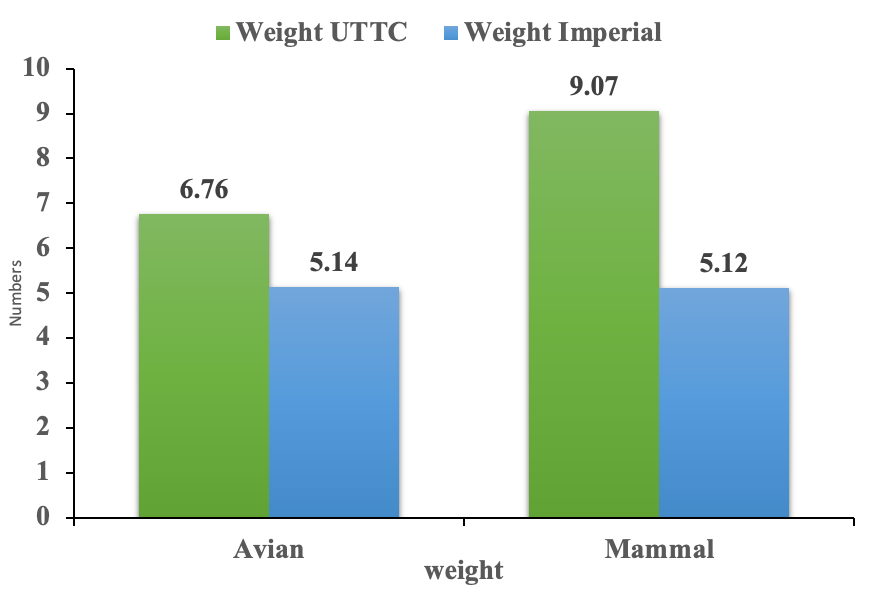
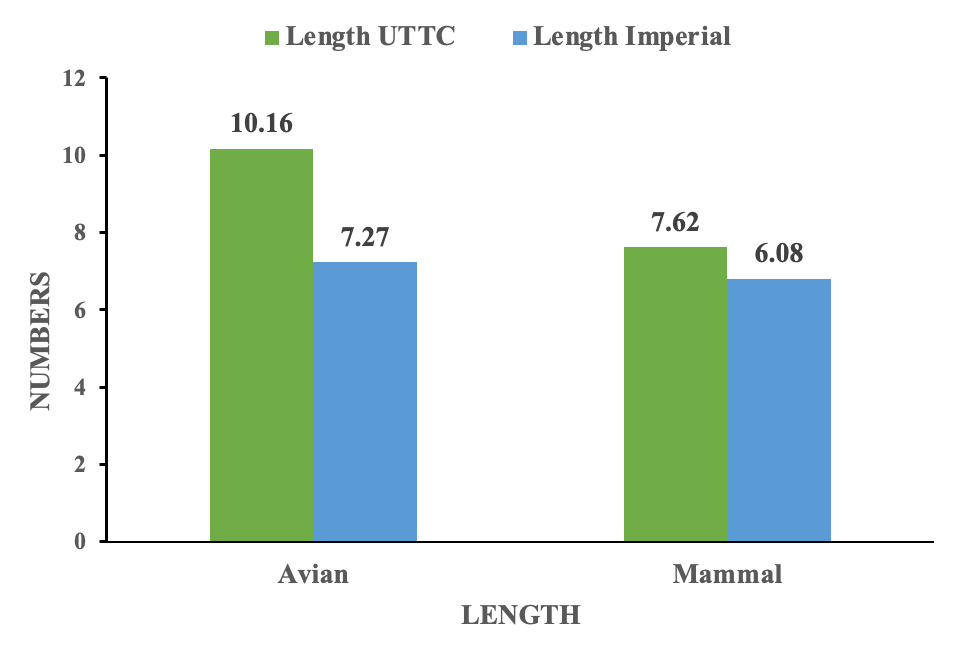
A total of 45 pellets were collected from the two study sites: 19 at UTTC and 26 at Imperial. Pellets were collected at UTTC from June to July 2021. UTTC pellets consisted of 84% mammal and 16% avian prey types. Imperial pellets consisted of 38% mammal and 62% avian prey types. The average weights of mammal and avian pellets at UTTC was 9.07 g and 6.76 g respectively. The average weights of mammal and avian pellets at Imperial was 5.12 g and 5.14 g respectively. The average length of mammal and avian pellets at UTTC was 7.62 cm and 10.16 cm respectively. The average length of mammal and avian pellets at Imperial was 6.08 cm and 7.27 cm respectively. There was no significant difference between the weights (p-value = 0.42) and lengths (p-value = 0.11) of avian pellets at both sites. There was a significant difference between the weights of mammal pellets at both study sites (p-value = 0.03). There was no significant difference between the lengths of mammal pellets at both study sites (p-value = 0.16). There was a significant difference between the prey types of pellets at both study sites (p-value = 0.0021). (Figure 1)
Discussion
I failed to reject my hypothesis that owls utilize different prey types at different locations during nesting and fledgling times. Owl diets consisted of more mammal than avian prey at the UTTC location, where the owls had young fledglings that were in hunting training. The nesting owl diets at the Imperial location consisted of more avian prey than mammal. The reasons for this difference maybe that the prey communities were different at each location and fledgling foraging practice may target easier prey.
Great horned owls are very skilled hunters and can help maintain rodent population sizes at low levels without the use of chemicals (Zabel et al. 1995). Currently, UTTC has a Richardson’s ground squirrel infestation and this method of control using owls can be used by installing nest boxes, providing owls a safe quiet place to go to during the day. Owls will reside in a location if they feel comfortable and unbothered. UTTC has locations that would be perfect for these nest box installations. This method of rodent control can be a biological alternative to chemicals, enhancing the safety of other wildlife and families located on the UTTC campus.
In Native American cultures owls are both feared and embraced. Many believed that owls could be “witches” or “bad medicine” that shapeshifted so they could fly silently through the night to cast spells on people while they slept and were vulnerable to spiritual forces. The main belief is that to see an owl means death will come to your family. Due to their horn-like tufts, great horned owls were seen as the most uncanny and dangerous of owls. Not all tribes believed owls were a bad omen. Instead, they sought spiritual help from owls in their healing practices. Some holy people believed that the owl had very soft and gentle ways, like the softness of an owl’s feather. Due to these sensitive beliefs and traditions the installation of nest boxes will need to be carefully considered.
For future research I would like to expand my location sites to areas all over Bismarck and Mandan. I would also like to get more detailed information in the prey species. Determining specific prey species by bone and feather identification and DNA analysis.
References
Courant FB, David B, Laurin B, Chaline J. 1997. Quantification of cranial convergences in arvicolids (Rodentia). Biol. J. Linn. Soc. 62: 505–517.
Errington PL, Hamerstrom F, Hamerstrom FN. 1940. The great horned owl and its prey in north-central United States. Iowa State University, 227 (24): 759-850.
Faanes CA. 1983. Breeding birds of wooded draws in western North Dakota. USGS Northern Prairie Wildlife Research Center. 21: 173–87. https://digitalcommons.unl.edu/usgsnpwrchttps://digitalcommons.unl.edu/usgsnpwrc/21.
Hunter BD, Rohner C, and Currle DC. 1997. “Mortality in fledgling great horned owls from black fly hematophaga and leucocytozoonosis.” Journal of Wildlife Diseases 33 (3): 486–91. https://doi.org/10.7589/0090-3558-33.3.486.
Johnsgard PA. 1988. North American owls: biology and natural history. 46: 7–295. http://digitalcommons.unl.edu/johnsgardhttp://digitalcommons.unl.edu/johnsgard/46.
Kullberg C. 1995. “Strategy of the pygmy owl while hunting avian and mammalian prey.” Ornis Fennica. Vol. 72.
Minor WF, Minor M, Ingraldi MF. 1993. Nesting of red-tailed hawks and great horned owls in a central New York urban/suburban area (Anidamiento de Buteo jamaicensis y de Bubo virginianus en un area urbana/suburbana de la parte central de New York). Journal of Field Ornithology, pp. 433-439.
Murphy RK. 1997. Importance of prairie wetlands and avian prey to breeding great horned owls (Bubo virginianus) in northwestern North Dakota. In: Duncan, James R.; Johnson, David H.; Nicholls, Thomas H., eds. Biology and conservation of owls of the Northern Hemisphere: 2nd International symposium. Gen. Tech. Rep. NC-190. St. Paul, MN: US Dept. of Agriculture, Forest Service, North Central Forest Experiment Station. 286-298. (Vol. 190).
Orians G, Kuhlman F. 1956. Red-tailed hawk and horned owl populations in Wisconsin. The Condor, 58(5), pp.371-385.
Tomazzoni AC, Pedó E, Hartz SM. 2004. Food habits of great horned owls (Bubo virginianus) in the breeding season in Lami Biological Reserve, southern Brazil. Ornitologia Neotropical, 15(2).
Trejo A, Kun M, Sahores, M, Seijas S. 2005. Diet overlap and prey size of two owls in the forest-steppe ecotone of southern Argentina. Ornitologia Neotropical, 16(4).
Wasser SK, Hunt KE. 2005. Noninvasive measures of reproductive function and disturbance in the barred owl, great horned owl, and northern spotted owl. Annals of the New York Academy of Sciences, 1046(1), pp.109-137.
Yom-Tov Y, Wool D. 1997. Do the contents of barn owl pellets accurately represent the proportion of prey species in the field?. The Condor, 99(4), pp.972-976.
Zabel CJ, McKelvey K, Ward Jr, JP. 1995. Influence of primary prey on home-range size and habitat-use patterns of northern spotted owls (Strix occidentalis caurina). Canadian Journal of Zoology, 73(3), pp.433-439.
Zalewski A. 1994. Diet of urban and suburban tawny owls (Strix Aluco) in the breeding season. Journal of Raptor Research 28 (4): 246–52.
About the Author

I am from the Turtle Mountain Band of Chippewa Reservation in North Dakota and the mother of two beautiful children that inspired my will to succeed. Growing up in a small but beautiful town of Belcourt on my father’s farm, I have always been connected to nature and animals. At the age of 27, I decided to go back to school to start a career. I first enrolled in the Culinary Arts program at United Tribes Technical College in Bismarck in 2015 but quickly realized that was not my true career passion. I learned about the Environmental Science and Research program and decided to enroll because I knew it would open up so many opportunities for me and my two children. While in the Environmental Science and Research Program, I conducted two independent research projects. My first involved tornado debris signatures to determine plant phenology and my second involved monitoring owl diets and their reproduction phases in the Bismarck area. After graduating with my bachelor’s degree in Environmental Science and Research I plan to start my career search somewhere in my education field. I do plan on continuing my education later in life. I would like to go back to school to get my master’s degree in wildlife biology or ornithology. I want my kids to see the hard work and dedication I put into following my dream with my desired career. I would also like to acknowledge my faculty advisors, Mandy Guinn and Alicia Andes. They were so motivational and great mentors throughout my years in college.
• • •
Enjoyed this story? Enter your email to receive notifications.
