By Robert Sam
United Tribes Technical College

Abstract
Agricultural pesticides are the primary application used for preventing pests and disease among crops. With a growing population and a corresponding need for sustainable food production, pesticides use has been on a steady increase. Despite their beneficial role in pest and disease suppression, however, pesticides have also been shown to have detrimental impacts on ecosystems. One primary area of interest is the impact pesticides have on soil ecology. Studies have reported decreases in growth, respiration, and nutrient availability for soil microbial communities. We wanted to observe an agrochemical’s effect on the soil microbial communities at different depths. I hypothesized that the agrochemical will have no significant difference on the soil microbial community respiration and metabolic rates at different soil depths. Samples were collected from two research plots at the Northern Great Plains Agricultural Research Station in Mandan, North Dakota during the summer of 2021. The first plot was a USDA certified organic plot. The second was an experimental plot that had multiple seasons of a controlled herbicide application. During the 2021 growing season the second plot was sprayed with crop oil and Jackhammer, a water conditioning agent. From each sample plot, two different soil depths were collected: 2.54-12.7 cm for the top layer and 20.32-25.4 cm for the subsoil. In total, 20 samples were collected from both plots. To test soil microbial activity and community diversity, we used the Solvita CO2 burst test and Biolog Ecoplates. Results showed the herbicide had no influence on microbial diversity or respiration rates. There was no significant difference in the Shannon Weiner diversity test (p-value = 0.085). There was a slight significant difference in Mcintosh evenness (p-value = 0.044). For species richness there was a significant difference (p-value = 0.033). A PCA analysis was conducted as well. The first principal component explained 57.85% of the variation in the data and second explained 19.67%. The PCA resulted in no significant groupings amongst treatment or depth. Although there is no evidence in this study to show that agrochemicals affect soil microbial communities, it is important to understand that soil and the microbes within are very important for agriculture and life itself.
Introduction
Pesticide use has increased through the years, corresponding with increased produce consumption by humans (Zhang et al. 2011). Pesticides are substances that are used to control nuisances to crops. Pesticides are used in agriculture to prevent pests such as insects, rodents, fungi, nematodes and bacteria from interfering with crops. In addition, Sun et al. (2018) noted that pesticides are also used to prevent disease among crops such as West Nile virus and Lyme disease. Therefore, agriculture heavily relies on pesticides and agrochemicals, but these have negative impacts on soil ecology (Anu & Gosal 2011).
Pesticides can impact soil ecology because they are applied several times during the growing season with some chemicals penetrating the top soil (Van Der Werf 1996). Pesticide use has created widespread problems for the environment including pollution (Arora & Sahni 2016). In addition, suppressed growth, respiration, and activity of soil bacteria are also negative impacts from pesticide use (Omar & Abd-Alla 1992).
Soil bacteria are a vital aspect for healthy soil. They form mutualistic relationships with plants to provide needed nutrients. For example, nitrogen fixation is a process that benefits both plants and bacteria. Bacteria provides the plant necessary nitrogen for processing proteins. The plant provides bacteria carbon and a secure environment in the roots (Dixon & Kahn 2004). Jacobsen & Hjelmso (2014) studied susceptibility of nitrification processes to pesticides. Bayo-Sanchez et al. (2002) reported that pesticide use is concerning because of the impacts to soil ecology, plants, water, sediment, and air. The application site and downwind areas are high zones for inhalation of airborne pesticides immediately after application and can reside in the area for up to three days. In addition, wildlife can reach the contaminated zones where pesticide application has occurred and come in contact with the soil or ingest the crops. Chi-Chu (2010) reported impacts of various pesticides on the health and the growth of both plants and soil microorganisms.
My objective was to examine the effects of an agrochemical on soil microbial communities at different soil depths in an agricultural landscape. This was accomplished through an examination of respiration and metabolic rates of soil microbes and their communities at different soil depths for pesticide and non-pesticide treated agricultural fields. I hypothesized that the agrochemical will have no significant difference on the soil microbial community respiration and metabolic rates at different soil depths.
Methods
My study sites were located at the Northern Great Plains Research Laboratory in Mandan, North Dakota. I obtained soil samples from two plots within my study site. Plot 1 was 360 ft x 220 ft (109 x 67 m) and Plot 2 was 690 ft x 220 ft (210 x 67 m). My control was Plot 1, which was a United States Department of Agriculture (USDA) certified organic field planted with Kernza, a variety of intermediate wheat-grass Thinopyrum intermedium. Plot 2 was my experimental plot, planted with a variety of different vegetation and subjected to agrochemical treatment: crop oil at 1 gallon per 100 gallons, Jackhammer conditioning agent at 1 gallon/100 gallons, and Shredder herbicide at 1 gallon/100 gallons. The pesticide was applied May 17, June 17, and June 22, 2021. I sampled soils on July 13 and July 19, 2021. Ten random locations for each plot (total = 20) were obtained using an online random number generator. Soil samples were obtained from each random location within the plot at two different depths: 2.54-12.7 cm for the top layer and 20.32-25.4 cm for the subsoil.
The soil samples were processed at United Tribes Technical College (UTTC). First, the soil samples were completely dried in an oven at 35 Celsius. All samples were sieved and to collect 2mm organic matter. Microbial respiration rate and microbial community diversity tests were conducted on the organic matter.
Microbial respiration rates were measured using the Solvita CO2 burst test. 2mm of dried soil samples are measured with a 30cc volume scoop and 9 cc of water was dispersed on the soil. The soil samples were then sealed and left to incubate at room temperature. The Solvita CO2 burst test was looking at the respiration of the soil microbes after they were reactivated by rewetting and sealed in an air tight container for 24 hours. The numbers used for measurements are based off of the Solvita analog color chart provided.
Microbial community diversity was measured using Biolog Ecoplate, which consisted of 96 wells with 3 duplicate carbon substrates. First, I completed a serial dilution of the organic matter to and then pipetted 100
l into each ecoplate well. Next the plates were incubated at room temperature for 120 hours. Finally, optical density readings were recorded every 24 hours from the start of incubation until 120 hours. Optical density readings showed the color development in each ecoplate well, which would indicate that metabolic activity occurred. At 120 hours each ecoplate optical density readings were taken and averaged out for 120 hours. This gave the average well color development (AWCD) for each plate. This was the maximum time for growth and utilization of carbon sources. To analyze metabolic and respiration activity of the microbial communities, I used an ANOVA to determine if there were any significant differences between average well color development and respiration rates for both treatment and soil depth. A community level physiological profiling (CLPP) method was used to analyze the microbial community data as well as a principal component analysis (PCA). The PCA analysis was constructed using carbon type, depths, and all soil sample utilization of carbon type. CLPP consisted of the following analyses: Shannon Weiner diversity, Mcintosh evenness and a richness index. Shannon Weiner diversity is a measurement of species diversity. Mcintosh evenness is a measurement of species evenness and richness is a measurement of species richness. I used ANOVA to determine if there were any significant difference between herbicide treatment and soil depths from the diversity, evenness, and richness analyses.
Results
AWCD showed that there was very slight significance between organic vs pesticide color development but this was barley less than .05. For the diversity indices, Mcintosh evenness and species richness were significant but evenness was only slightly significant with a value of 0.044. The same goes for richness, where its p-value is 0.033 so it is worth mentioning it was more significant than evenness. Figure 1 shows AWCD for all samples measuring incubation time versus the optical density. AWCD numbers are based off of the average of optical density readings at 120 hours. Figure 2 shows the same thing except I grouped my samples by treatment type using the same metrics.
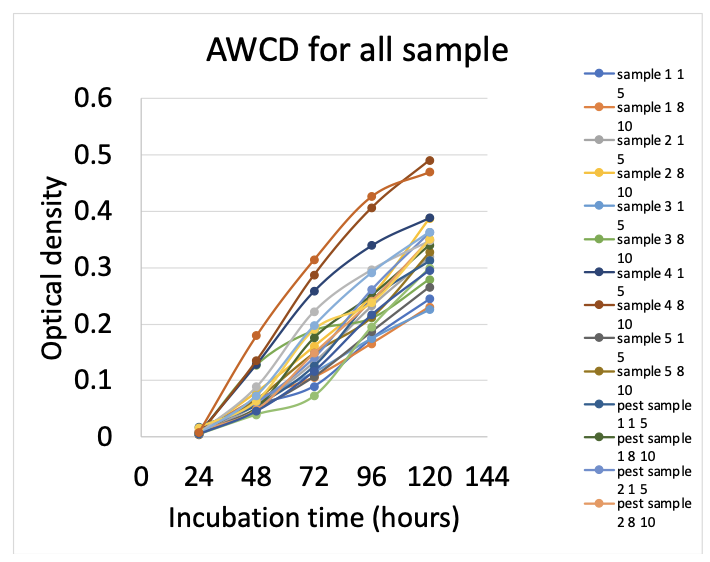
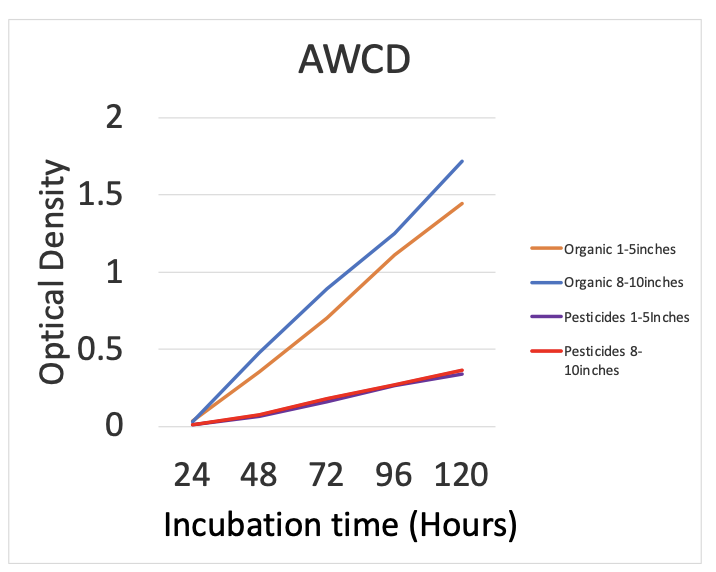
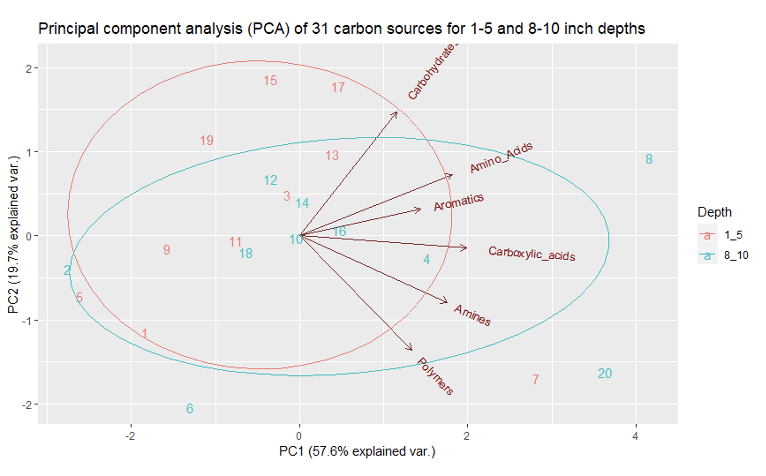
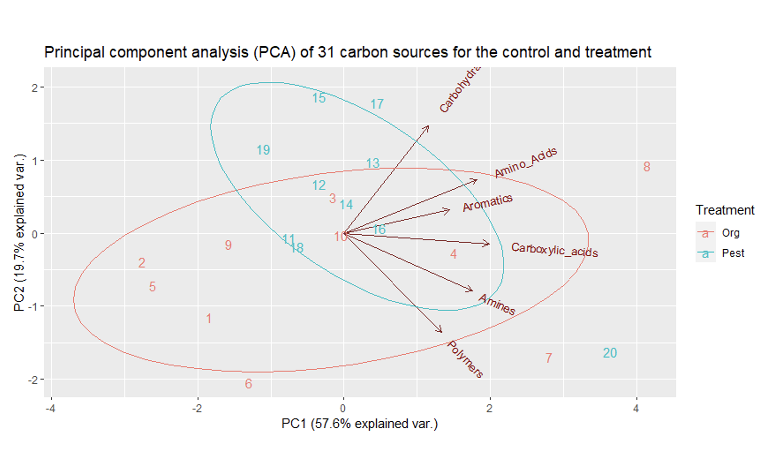
There was a slight but significant difference between AWCD for treatment and soil depths (p-value = 0.0429). there was no significant difference between the Shannon Weiner diversity index for treatments and soil depths (p-value = 0.085). There was a slight but significant difference between the Mcintosh evenness index for treatment and soil depths (p-value = 0.044). There was a significant difference between the richness index for treatment and soil depths (p-value = 0.033). A PCA analysis was also performed for both treatment and depth. The first principal component explained 57.85% of the variation in the data and second explained 19.67% (figure 3 & 4). The PCA resulted in no significant groupings amongst treatments or depth.
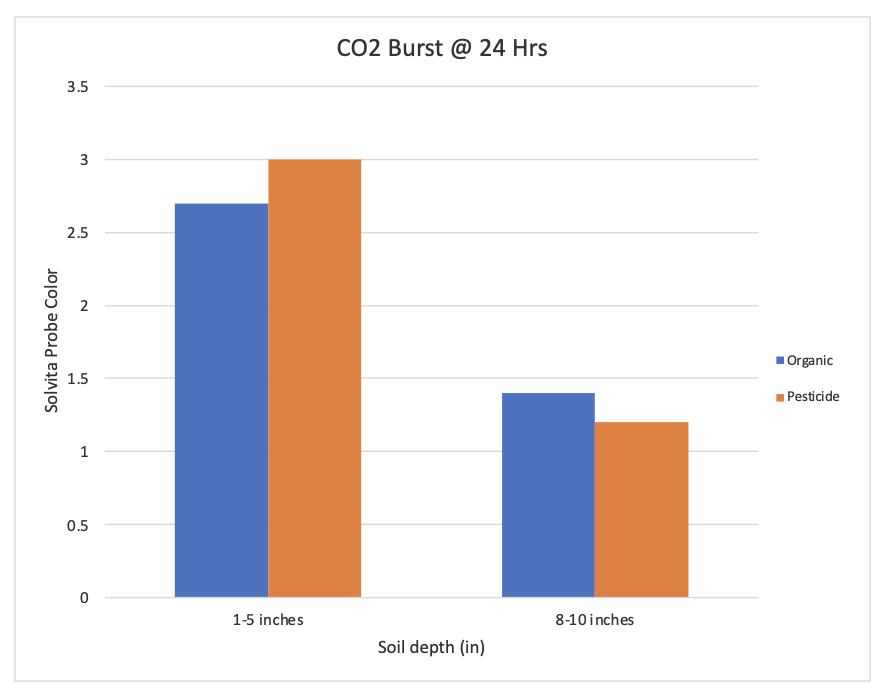
Respiration rate of microbial communities was greater at the 2.54-12.7 cm of top soil compared to deeper depths at 20.32-25.4 cm (Figure 5). There was no significant difference between respiration rates of microbial communities between organic vs herbicide plots.
Discussion
Based on the results of this research, I failed to reject my hypotheses. The Jackhammer herbicide did not influence microbial community diversity or respiration rates. Richness was the only factor that was influenced by either herbicide, depth, or a combination of both. This could be an outcome of the herbicide taking effects on the species that reside in the soil and how far the herbicide leeches into the soil. In other words, at different depths, no matter the treatment species, richness varied. Respiration rate was influenced by depth of the soil. The microbial community at deeper soil depths had lower respiration rates. The respiration rates being lower at different soil depths indicates anaerobic soil microbes.
Some limitations of this project included a small sample size with one location, consideration of the Kernza growing season, and weather. During the study there was a drought, which may have impacted the results. Furthermore, I only sampled the one soil type and tested one herbicide.
References
Anu, K. & Gosal, S. K. (2011). Effect of pesticide application on soil microorganisms, Archives of Agronomy and Soil Science, 57(6), 569-596. DOI: 10.1080/03650341003787582
Arora, S., & Sahni, D. (2016). Pesticides effect on soil microbial ecology and enzyme activity- an overview. Journal of Applied and Natural Science, 8(2), 1126-1132. https://doi.org/10.31018/jans.v8i2.929
Bayo-Sanchez, F., Baskaran, S., Kennedy, R, I. (2002). Ecological relative risk (EcoRR): another approach for risk assessment of pesticides in agriculture. Agriculture, Ecosystems & Environment, 91(1), 37-57. https://doi.org/10.1016/S0167-8809(01)00258-4
Chi-Chu, L. (2010). Effect of pesticides on soil microbial community. Journal of Environmental Science and Health, Part B, 45(5), 348-359, DOI: 10.1080/03601231003799804
Dixon, R. & Kahn, D. (2004). Genetic regulation of biological nitrogen fixation, Nature Reviews Microbiology, 2, 621-631. https://doi.org/10.1038/nrmicro954
Feld, L. et. al. (2015). Pesticide side effects in an agricultural soil ecosystem as measured by amoa expression quantification and bacterial diversity changes. PloS one, 10(5) e0126080. https://doi.org/10.1371/journal.pone.0126080
Gilliom, J, R. (2007). Pesticides in U.S. streams and groundwater. Environmental Science And Technology, 41(10), 3408-3414. https://pubs.acs.org/doi/pdf/10.1021/es072531u
Jacobsen, S, C., & Hjelmso, H, M. (2014). Agricultural soils, pesticides and microbial diversity, Current Opinion in Biotechnology, 27, 15-20. https://doi.org/10.1016/j.copbio.2013.09.003
Johnsen, K., Jacobsen, C.S., Torsvik, V. et al. (2001). Pesticide effects on bacterial diversity in agricultural soils – a review. Biology and Fertility of Soils 33, 443–453. https://doi.org/10.1007/s003740100351
Kaufman, D. D. (1974). Degradation of pesticides by soil microorganisms. Pesticides in Soil and Water, 1(1). https://doi.org/10.2136/1974.pesticides.c8
Mahmood, I., Imadi S.R., Shazadi K., Gul A., Hakeem K.R. (2016) Effects of Pesticides on Environment. In: Hakeem K., Akhtar M., Abdullah S. (eds) Plant, Soil and Microbes. Springer, Cham. https://doi.org/10.1007/978-3-319-27455-3_13
Maroni, M., Fanetti, A, C., & Metruccio, F. (2006). Risk assessment and management of occupational exposure to pesticides in agriculture. La Medicina Del Lavoro, 97(2), 430-437. https://europepmc.org/article/med/17017381
Niewiadomska A., & Klama K., (2005). Pesticide side effect on the symbiotic efficiency and nitrogenase activity of rhizobiaceae bacteria family. Polish Journal of Microbiology, 54(1), 43-44.
Omar, S.A., & Abd-Alla, M.H. (1992). Effect of pesticides on growth, respiration and nitrogenase activity of azotobacter and azospirillum. World J Microbiol Biotechnol, 8, 326-328. https://doi.org/10.1007/BF01201891
Prashanth, P., Vijay, K. Y. A., & Manjula, P. (2012). Pesticide applications-Threat to ecosystems, Journal of Human Ecology, 32(1), 37-45. https://doi.org/10.1080/09709274.2010.11906319
Schuster, E. & Schroder, D. (1990). Side-effects of sequentially- and simultaneously- applied pesticides on non-targeted soil microorganisms: laboratory experiments. Soil Biology and Biochemistry, 22(3), 375-383. https://doi.org/10.1016/0038-0717(90)90116-H
Shakerkhatibi, M., Mosaferi, M., Asghari Jafarabadi, M., Lotfi, E., & Belvasi, M. (2014). Pesticides residue in drinking groundwater resources of rural areas in the northwest of iran. Health promotion perspectives, 4(2), 195–205. https://doi.org/10.5681/hpp.2014.026
Sun, S., Sidhu, V., Rong, Y., & Zheng, Y. (2018). Pesticide pollution in agricultural soils and sustainable remediation methods: a review. Current Pollution Reports, 4, 240–250. https://doi.org/10.1007/s40726-018-0092-x
Trasar-Cepeda, C., Leiros, M, C., Seoane, S., & Gil-Sotres, F. (2000). Limitations of soil enzymes as indicators of soil pollution. Soil Biology and Biochemistry, 32, 1867-1875. https://doi.org/10.1016/S0038-0717(00)00160-7
Van Der Werf, H.M.G. (1996). Assessing the Impact of Pesticides on the Environment. Agriculture, Ecosystems and Environment 60(1), 81-96. https://doi.org/10.1016/S0167-8809(96)01096-1
Weston, P, D., You, C, J., & Lydy, J, M. (2004). Distribution and toxicity of sediment-associated pesticides in agriculture-dominated water bodies of California’s central valley. Environmental Science & Technology, 38(10), 2752-2759. DOI: 10.1021/es0352193
Yasmin, S. & D’Souza, D. (2010). Effects of pesticides on the growth and reproduction of earthworm: a review. Status, Trends, and Advances in Earthworm Research and Vermitechnology, 2010, 9. https://doi.org/10.1155/2010/678360
Yen, J, H., Lin, K, H., & Wang, Y, S. (2000). Potential of the insecticides acephate and methamidophos to contaminate groundwater. Ecotoxicology and Environmental Safety, 45(1), 79-86. https://doi.org/10.1006/eesa.1999.1846
Zhang, W., Jiang, F., & Ou, J. (2011). Global pesticide consumption and pollution: with China as a focus. International Academy of Ecology and Environmental Sciences, 1(2), 124-144. https://pdfs.semanticscholar.org/6e82/99fcbc98b76637430502c0398d558640c348.pdf
About the Author

I am a proud enrolled member of the Shoshone-Paiute Tribe of Owyhee Nevada. Currently finishing up my bachelor’s degree in Environmental Science and Research at United Tribes Technical College in Bismarck, North Dakota, I plan on continuing my education to achieve a master’s degree in a program related to my interests. I am currently a Year-round Intern at Sandia National Laboratories working in their geophysics department. I plan on continuing my work with Sandia National Laboratory and hopefully work there full-time once I receive my master’s degree. I would not have made it this far without the support of my family, friends, and supportive faculty, including my advisors, Dr. Alicia Andes-Buysse and Dr. Mandy Guinn.
