
By S’Nya Sanchez, Gurjot S. Dhaliwal, Mandy Guinn, Ram Krishna Hona
Abstract
Wheatgrass can be grown indoors throughout the year irrespective of climate or seasons. The iron content in wheatgrass can vary depending on the growing conditions and harvesting methods. This study looks at the effect of iron concentration in soil on the wheatgrass plant when applying iron solutions of different concentrations. The spectrophotometric (colorimetric) analysis is performed to analyze the iron amount by the reaction between Fe2+ ions and 1,10-phenanthroline. The study shows that the highest amount of iron is found in the grass to which 0.05 Fe2+ solution was watered, and the lowest amount of iron was found in the grass to which 5 x 10-6 M Fe2+ solution was watered. This indicates that wheatgrass absorbs a higher amount of iron while a higher concentration of iron is present in the soil.
Introduction
Wheatgrass is the young grass of the wheat plant, Triticum aestivum. It is widely consumed as a dietary supplement due to its numerous health benefits. Scientific studies show that It is rich in nutrients such as vitamins, minerals, and antioxidants that can help boost overall health[1]. It is high in antioxidants such as flavonoids and phenolic acids, which can help protect against oxidative stress and inflammation[2]. Regular consumption of its juice may help lower cholesterol levels in people with high cholesterol[3].Consuming wheatgrass juice may help increase the number of white blood cells, which play a crucial role in fighting infections and diseases.[4] Some studies have suggested that wheatgrass may have anti-cancer properties. According to a study published in the Journal of Clinical Oncology, consuming wheatgrass juice may help improve the quality of life in people undergoing chemotherapy for breast cancer [4, 5]. It also contains other nutrients such as vitamin C and chlorophyll. Vitamin C can help the body absorb and utilize minerals [6], while chlorophyll may help to enhance the oxygen-carrying capacity of red blood cells[7].
Wheatgrass is also a rich source of iron, an essential mineral that is necessary for the formation of red blood cells and the transportation of oxygen throughout the body. Iron is also important for maintaining a healthy immune system and supporting cognitive function. Iron is essential for the formation of red blood cells and the transportation of oxygen throughout the body. Consuming wheatgrass regularly may help prevent or alleviate symptoms of iron deficiency anemia, a condition that occurs when the body doesn’t have enough iron to produce sufficient red blood cells[8]. Iron deficiency or anemia can lead to symptoms such as fatigue, weakness, shortness of breath, and impaired cognitive function. Iron can be added to our body through wheat grass. The iron content in wheatgrass can vary depending on the growing conditions and harvesting methods, but it is generally considered to be a good source of iron. A 2015 study published in the Journal of Medicinal Plants Research found that wheatgrass contained approximately 68.45 mg/kg of iron. [9, 10].
Since wheatgrass can be grown indoors in any part of the planet throughout the year irrespective of climate and seasons; iron content can be controlled in the grass depending on the growing condition and harvesting methods, we studied the effect of regular watering the grass with iron solutions of different concentrations.
Experimental Preparation of iron solutions to water the wheatgrass
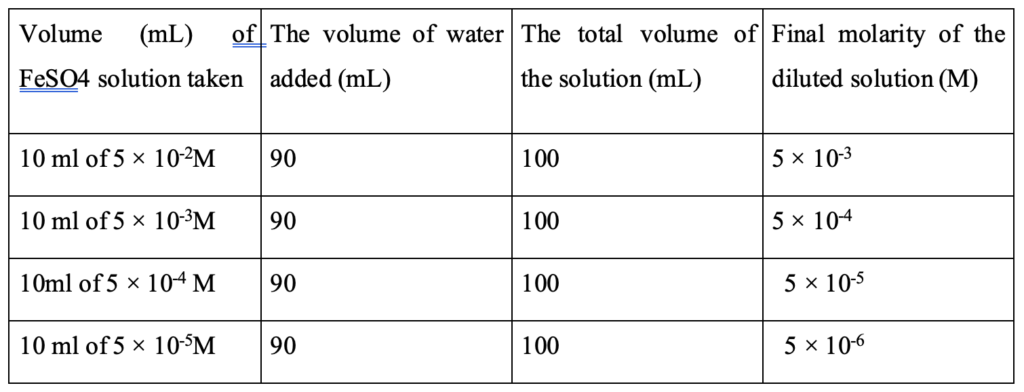
Iron solutions were prepared by dissolving FeSO4.7H2O in distilled water. The molar mass of FeSO4.7H2O is 278 and that of FeSO4 is 151.908. Here, 1.39 g of FeSO4.7H2O was dissolved in 50 mL of water in a 100 mL volumetric flask and then water was added up to 100 mL mark. The resulting solution was a 0.05 M FeSO4 solution containing 0.7595 g of it. Then it was diluted to the desired concentrations as in Table 1. These solutions were used for watering the wheatgrass.
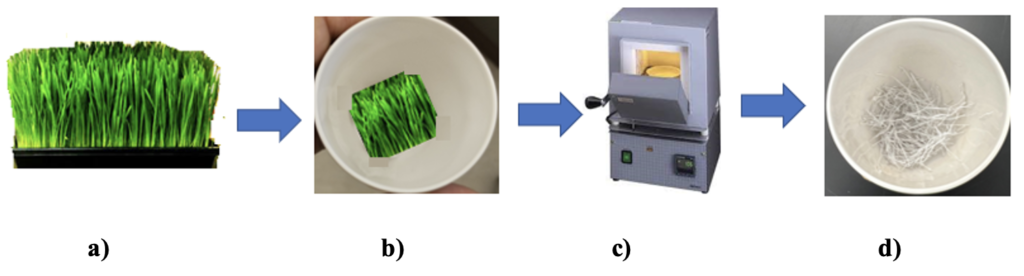
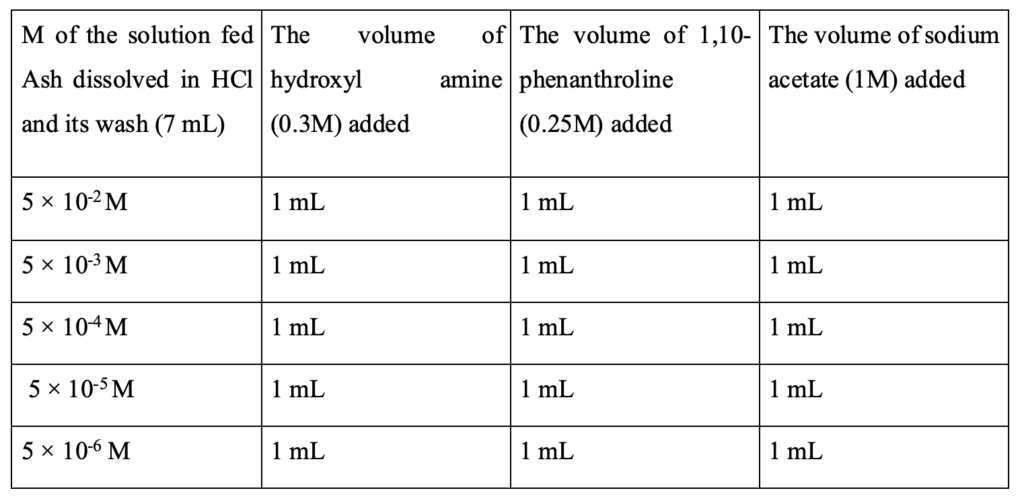
Wheatgrass growing process Wheat seeds were purchased online. 4 grams of wheat seeds were spread over powdered soil in each of the six trays. The seeds were covered by a thin layer of soil. Individual trays with seeds were numbered for identification and watered with iron solutions of different concentrations. Tray 1 was watered every day with pure/distilled water only. Tray 2 was watered with an iron concentration of 5 × 10-6 M and so on. Everyday each tray was watered with 10 mL of the solutions. The grass was grown in a growth chamber at 76 ⁰F for one week to 10 days. No direct sunlight was exposed to the seeds and plants. Wheatgrass starts growing after two days. It was ready for juice in one week to 10 days. The green leaves of the grass were cut and weighed for burning. Equal weights of grass from different cups were taken into separate porcelain crucibles and fired at 600 ⁰C for 12 hours in an ambient environment in a muffle furnace (Fischer brand). The grass turns into white ash as shown in Figure 1. The crucibles were allowed to cool down to room temperature. In each experiment or for each crucible, 10 drops of concentrated HCl were added to dissolve all the ash. It was diluted by adding 2 mL of distilled water to dissolve or mix all the ash in the crucible. The solution was transferred to a 10 mL volumetric flask. The crucible was washed with 2 mL of distilled water and the wash was transferred to the volumetric flask. The crucible washing was repeated one more time. The whole solution (about 7 mL) in the volumetric flask was then diluted to 10 mL (up to mark) and mixed well. In each of the solutions, hydroxyl amine, 1,10-phenanthroline, and sodium acetate were added and shaken well to mix it well. The solutions turned to bright orange color. The solution was filtered before UV absorption measurement.
Preparation of standard solution reagents for colorimetric (concentration) measurement
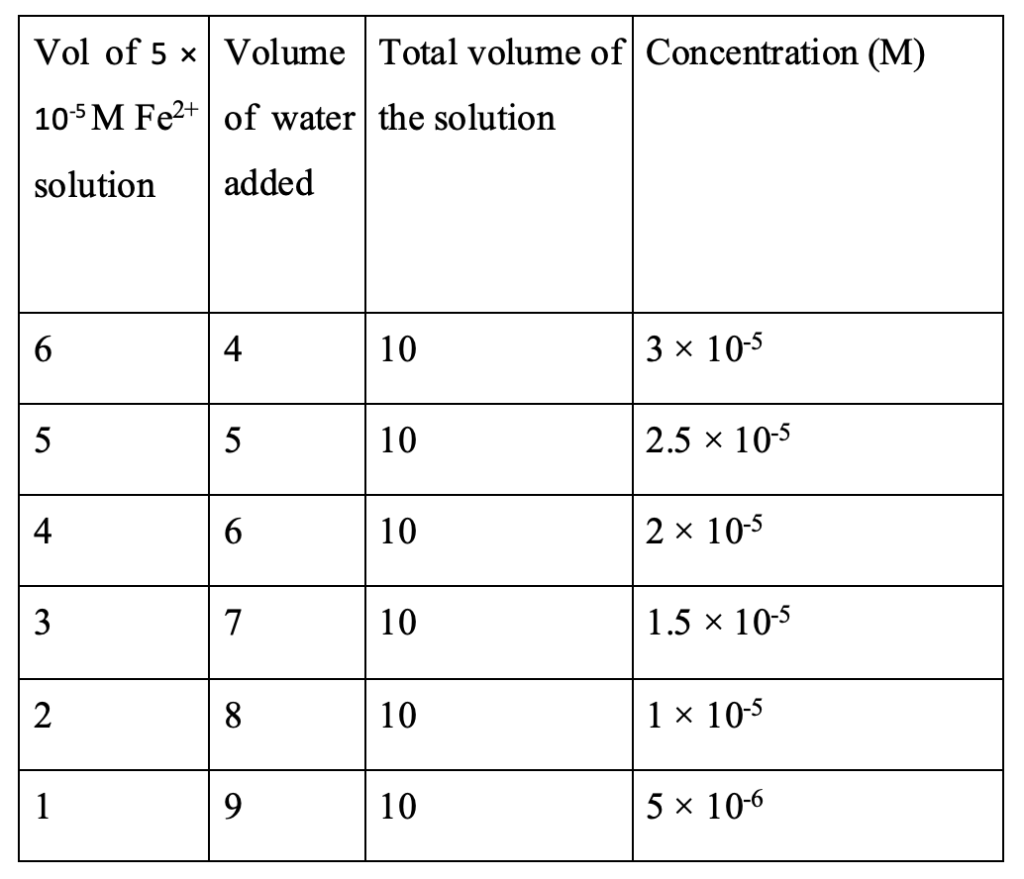
Aqueous solutions of 0.3 M hydroxylamine hydrochloride and 1.0 M sodium acetate were prepared. 0.25% 1,10-phenanthroline solution was prepared by dissolving 0.25g of it in 100 ml water. 1M acetate solution was prepared by dissolving 8.2 g of it in 100 ml of water. 0.3M hydroxyl amine was prepared by dissolving 2.0847 g of it in 100 ml water. Standard solutions were prepared from FeSO4.7H2O. First, 5 × 10-5 M solution was prepared as discussed above and then diluted to the desired concentrations as given in Table 2.
Preparation of blank
10 ml of blank solution is prepared by mixing 7 ml of water, 1 ml of hydroxyl amine, 1 ml of 1,10-phenanthroline and 1 ml of sodium acetate solutions.
UV absorption measurement
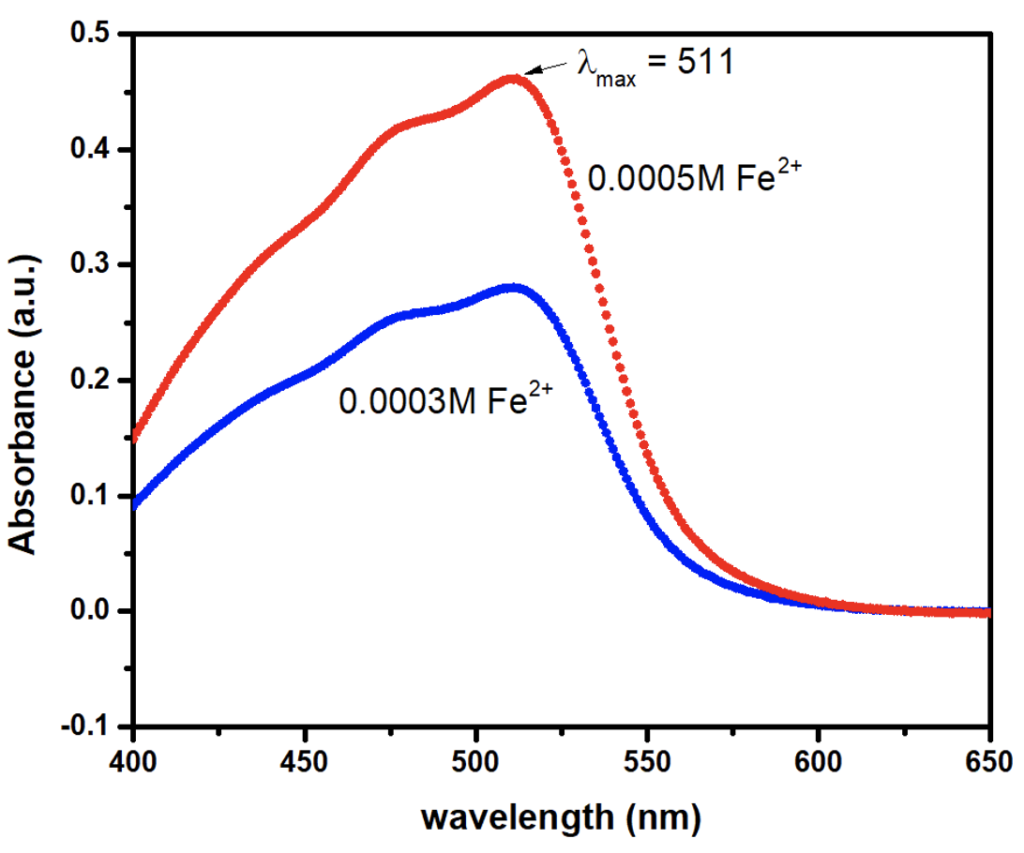
Genway UV vis and Evolution 1 UV vis were used for the measurement of UV absorption. The blank was used each time before measuring the UV absorption of the sample solutions. The λmax was first scanned at variable wavelength and at a single wavelength for standard solutions. Both the measurements showed the λmax at 511 nm. Then, all the measurements were accomplished at the same λmax.
Spectrophotometric analysis and quantitative determination
The iron amount in the wheatgrass was determined by colorimetric or spectrophotometric method. The wheatgrass was grown for one week. The green leafy part of the grass was cut, weighed, and burnt at 600 C.[11] The white ash obtained from burning was dissolved in Conc HCl. The acid solution may contain both Fe2+ and Fe3+ ions. Since the iron content in the grass is being determined in the form of Fe2+, all the Fe3+ ions need to be reduced to Fe2+. The reduction reaction can be accomplished by treating the solution with hydroxyl amine. The reduction reaction takes place as follows.
2Fe3+ (aq) + 2NH3OH– (aq) → 2Fe2+ (aq) + N2 (g) + 2H2O(l) + 4H+(aq)
1,10-phenanthroline is added to the solution. After addition of 1,10-phenanthroline, Fe2+ ions form a complex with three molecules of 1,10-phenanthroline. The complex formed by 1,10-phenanthroline and Fe2+, ferrous tris(1,10-phenanthroline) or [Fe(phen)3]2+, is a bright orange color.
Fe2+ + 3phen → [Fe(phen)3]2+
The λmax for the standard solution was found at 511 nm as shown in the figure 2. So, the absorbance of the colored complex is measured at 511 nm using a spectrophotometer. For the Fe2+ ion determination, a straight line of standard solutions was obtained which obeys Beer-Lambert’s law in the range of the given concentrations. The range for the validity of Beer Lambert’s law can be determined by plotting a calibration curve by measuring the absorbance values for a series of standard solutions of the complex being determined at a fixed wavelength of 511 nm. The curve so obtained is then used for the determination of concentration of unknown solutions from a measurement of its absorbance at the same wavelength.
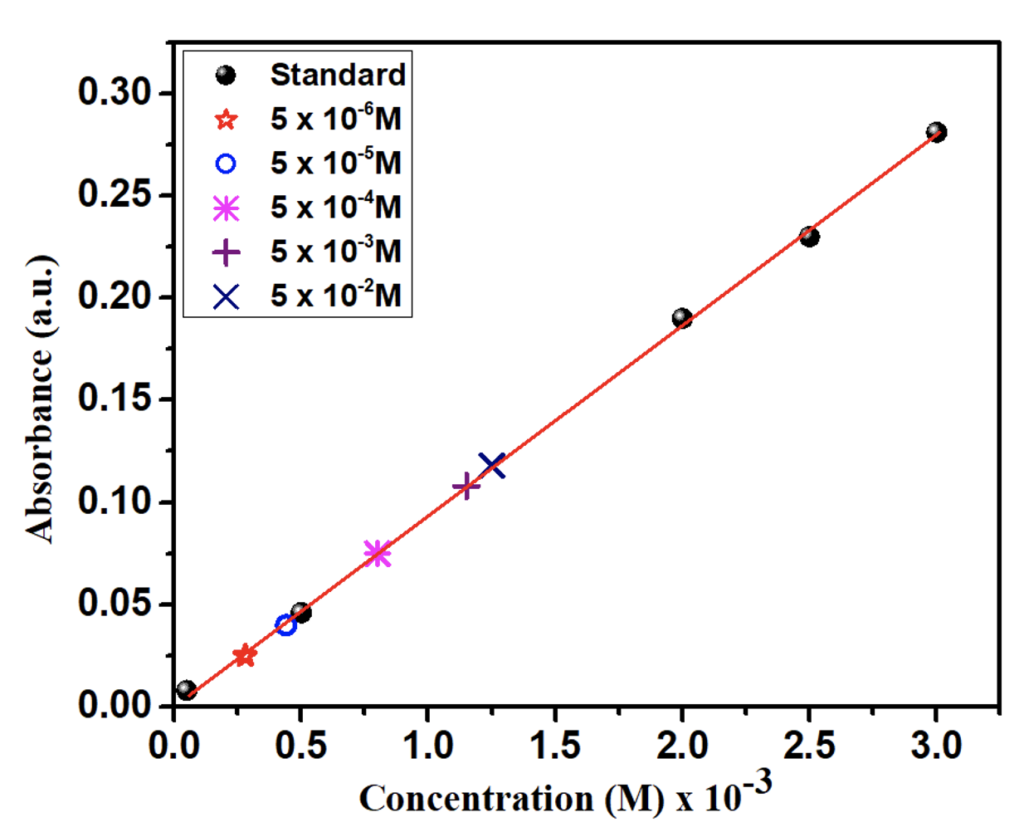
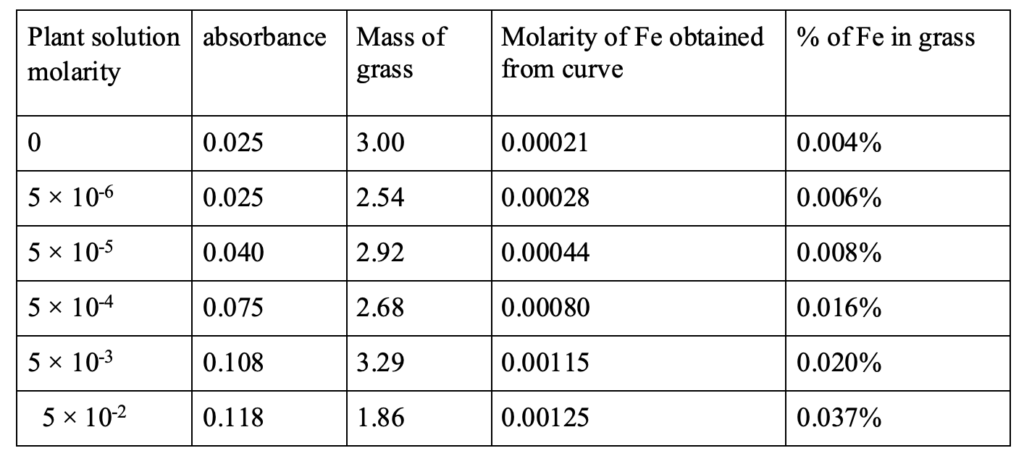
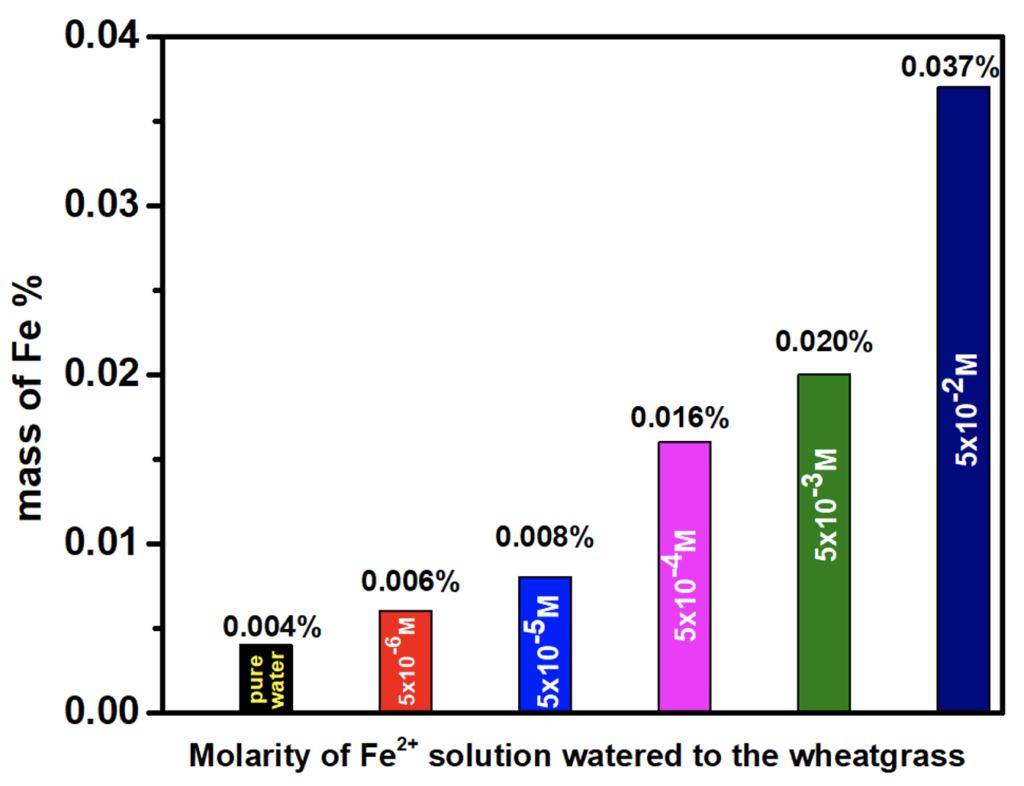
The relation between iron concentration in the wheatgrass and UV absorption is shown in figure 3. This figure also shows the relation of concentration of iron solution with UV absorption and concentration of Fe in wheatgrass. The overall relation of watering Fe2+ solution, UV absorbance, mass of iron in each wheatgrass is shown in Table 4.It is observed that the UV absorbance is the highest for 0.05 M watering solution and the lowest for 5 × 10-6 M. The absorbance goes down further when the grass is watered with pure water. The grass grown with the solution of lower iron concentration shows low amount of iron and that grown with higher concentrations shows higher iron amount. It demonstrates that the iron amount absorbed by the same amount of wheatgrass grown with solutions of different iron concentrations are different. The comparative total amount of iron found in different grass is shown in bar graph, figure 4. It indicates that the iron absorption by the wheatgrass is directly related to the amount of iron present in the soil. It is therefore revealed that iron concentration in wheatgrass juice can be increased naturally by adding iron salt solution in the soil before or during the grass’s growth.
Mass % calculation
The calculation performed to get mass was given as the following example:
The molarity is 1.25 x 10-3 M (from graph) for 0.05M Fe solution-fed grass.
It means,
1000 mL solution contains 1.25 x 10-3 mole of iron. (mole or 1.25 x 10-3 = mass/molar mass)
1000 mL solution contains 1.25 x 10-3 x 56 g (or 7.00 x 10-2 g) of iron.
10 mL of solution contains 7.00 x 10-4 g of iron.
10 mL of the solution is made of 1.86 g of wheat grass.
So, 1.86 g of wheatgrass contains 7.00 x 10-4 g of iron.
100 g of wheatgrass contain (7.00 x 10-2 / 1.86) or 0.038g of iron (or 3.8 mg) or 0.038%
Or, simply the calculation can be implied as
Mass = (M x 56 x 1/100) x 1/mass grass taken) x 100 %
= Fe per 100 g of wheatgrass
Conclusion
Iron concentration in wheatgrass juice can be controlled naturally by adding iron salt in soil in the solution form before or during the grass growing. If the soil has a higher amount of iron, the wheatgrass juice will have a higher amount of iron and vice versa. Though the iron content can depend on the grass growing environment and location, there may be a certain limit for the higher concentration of iron that is favorable for the good growth of the grass.
Acknowledgements:
This work is supported in part by the National Science Foundation Tribal College and University Program’s Instructional Capacity Excellence in TCUP Institutions (ICE-TI) award # 1561004, and TEA Center award # 1839895. We express gratitude to the program managers and review panels for project support. Additional support for the work came from ND EPSCOR STEM grants for research equipment. Permission was granted by United Tribes Technical Colleges (UTTC) Environmental Science Department to publish this information. The views expressed are those of the authors and do not necessarily represent those of United Tribes Technical College.
References
1. Parit, S.B., Dawkar, V. V., Tanpure, R. S., Pai, S. R., Chougale, A. D., Nutritional Quality and Antioxidant Activity of Wheatgrass (Triticum aestivum) Unwrap by Proteome Profiling and DPPH and FRAP assays. J. Food Sci., 2018. 83(8): p. 2127-2139.
2. Singh N., Anti-inflammatory and antioxidant effects of wheatgrass juice in chronic obstructive pulmonary disease patients: a randomized controlled trial. Indian J. Pharmacol, 2013. 45(2): p. 141-145.
3. Viju M., The effect of wheat grass powder supplementation on lipid profile and antioxidant status in response to high-fat diet-induced obesity in rats. J. Dietary Supplements. , 2017. 14(1): p. 1-16.
4. Avisar, A., Cohen, M., Katz, R., Shentzer Kutiel, T., Aharon, A., Bar-Sela, G.,, Wheatgrass Juice Administration and Immune Measures during Adjuvant Chemotherapy in Colon Cancer Patients: Preliminary Results. Pharmaceuticals (Basel), 2020. 13(6).
5. Bar-Sela, G., Tsalic, M., Fried, G., Goldberg, H.,, Wheat grass juice may improve hematological toxicity related to chemotherapy in breast cancer patients: a pilot study. Nutr. Cancer, 2007. 58(1): p. 43-8.
6. Hurrell, R. and I. Egli, Iron bioavailability and dietary reference values. Am. J. Clin. Nutr., 2010. 91(5): p. 1461s-1467s.
7. Ogawa, K., Hirano, K., & Kondo, M. , Heme oxygenase-1-dependent antioxidant effect of 3-hydroxymethyl chlorophyll a in human endothelial cells. . Free Rad. Bio. Med., 2012. 53(8): p. 1744-1752.
8. Mathur S., M.R., Kohli G.K., Therapeutic Use of Wheat Grass Juice for the Treatment of Anemia in Young Women of Ajmer City. Int. J. Nutr. Sci., 2017. 2(1): p. 1014.
9. Choudhary, D.R., Naithani, R., Panigrahi, I., Kumar, R., Mahapatra, M., Pati, H. P., Saxena, R., Choudhry, V. P., Effect of wheat grass therapy on transfusion requirement in beta-thalassemia major. Indian J. Pediatr., 2009. 76(4): p. 375-6.
10. Gharavi, M., Soltani, B. M., Shahsavari, G., & Ebrahimi-Mameghani, M. , The effect of wheatgrass juice on the transfusion requirement and quality of life of thalassemic patients. J. Med.Plants Res., 2015. 9(14): p. 462-466.
11. Vio T., Dhaliwal G.S., Guinn M., Hona R.K.,, Comparative Study of Iron Content in Apples of Different Brands or Places. Native Sci. Rep., 2022.
About the Authors
S’Nya Sanchez is a student enrolled at United Tribes Technical College. After completing an associate degree in science she is now pursuing a second associate degree in business. “I learned a lot during my first few years in the science program,” she writes, “where I enjoyed the support from my advisor, Mandy Guinn, and my professor, Ram Hona. Both helped me understand and learn more about what the science program had to offer. I was always welcomed in the lab and they both introduced me to new opportunities. I am grateful to have had them during my years in the Environmental Science degree program.”
Dr. Dhaliwal is faculty at intertribal Research and Resource Center
Mrs. Guinn is the department chair at Environmental Science Department
Dr. Hona is a faculty mentor at Environmental Science Department
Correspondence: rhona@uttc.edu
• • •
Published October 24, 2023
• • •
Liked this story? Enter your email to receive notifications.
